
Insights on symptoms, biology, and quality of life
By Yi-Wen Chen, PhD and Jean Mah, MD
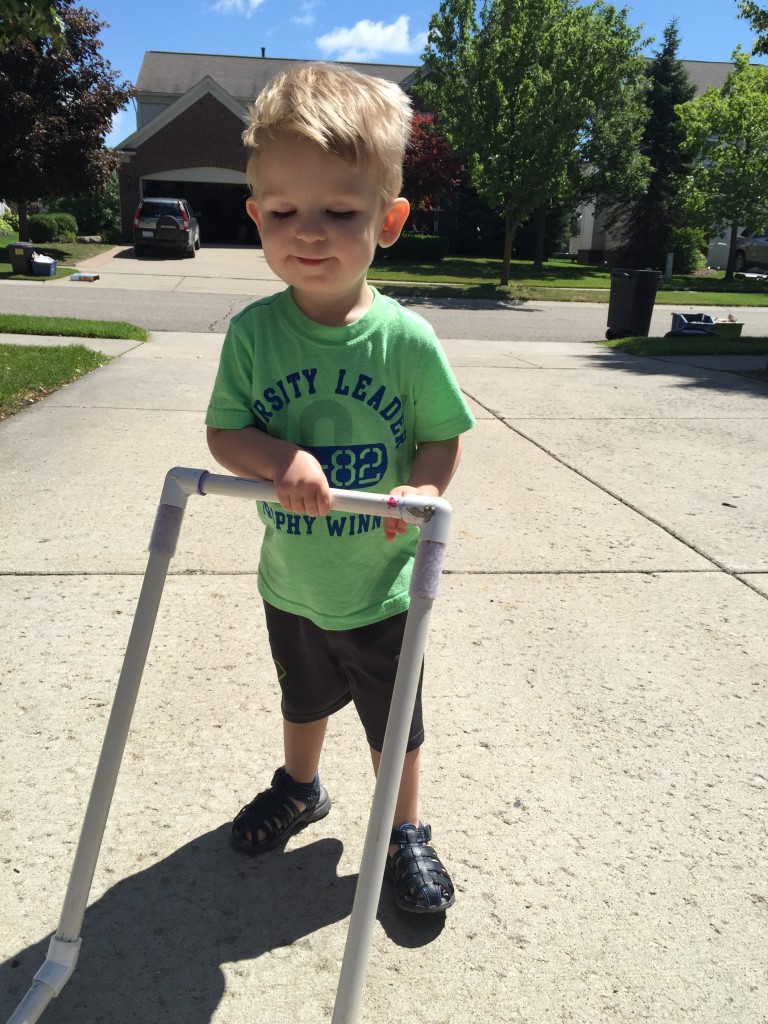
While most people with FSHD develop muscle weakness during late teen years or adulthood, about 4 percent of patients have early-onset FSHD. Patients with early-onset FSHD develop disease symptoms early in life (facial weakness before 5 years old and shoulder girdle weakness before 10 years old), have more severe muscle weakness, are more likely to need a wheelchair later in life, and have a higher chance of developing non-muscle symptoms (hearing loss, retinal vascular disease, developmental delays, and cognitive/intellectual disabilities).
FSHD is caused by aberrant expression of double homeobox protein 4 (DUX4) in patients’ cells. It is believed that patients with early-onset FSHD have genomic mutations that allow for a higher frequency of DUX4 expression in cells (associated with fewer D4Z4 repeats compared to late-onset patients), which contributes to the earlier and more severe disease symptoms.

In 2010, the FSH Society released a request for application (RFA) to study early-onset FSHD. In response to the RFA, our research team proposed to conduct a multicenter collaborative study on the clinical features, expression profiling, and quality of life of individuals with early-onset facioscapulohumeral muscular dystrophy.
The main goals of the study were to establish a standardized muscle testing protocol in children and young adults with early-onset FSHD, to better understand clinical phenotypes of early-onset FSHD, and to evaluate the impact of the disease on health-related quality of life and disability across different age groups. In addition, optional blood samples were collected for future biomedical research.
The studies were conducted by the Cooperative International Neuromuscular Research Group (CINRG), which was founded in 1999 at Children’s National Medical Center and has more than 30 neuromuscular clinical centers currently in the U.S., Canada, and beyond. Eleven CINRG sites participated in this study and had enrolled a total of 53 patients when enrollment was completed in early 2015.
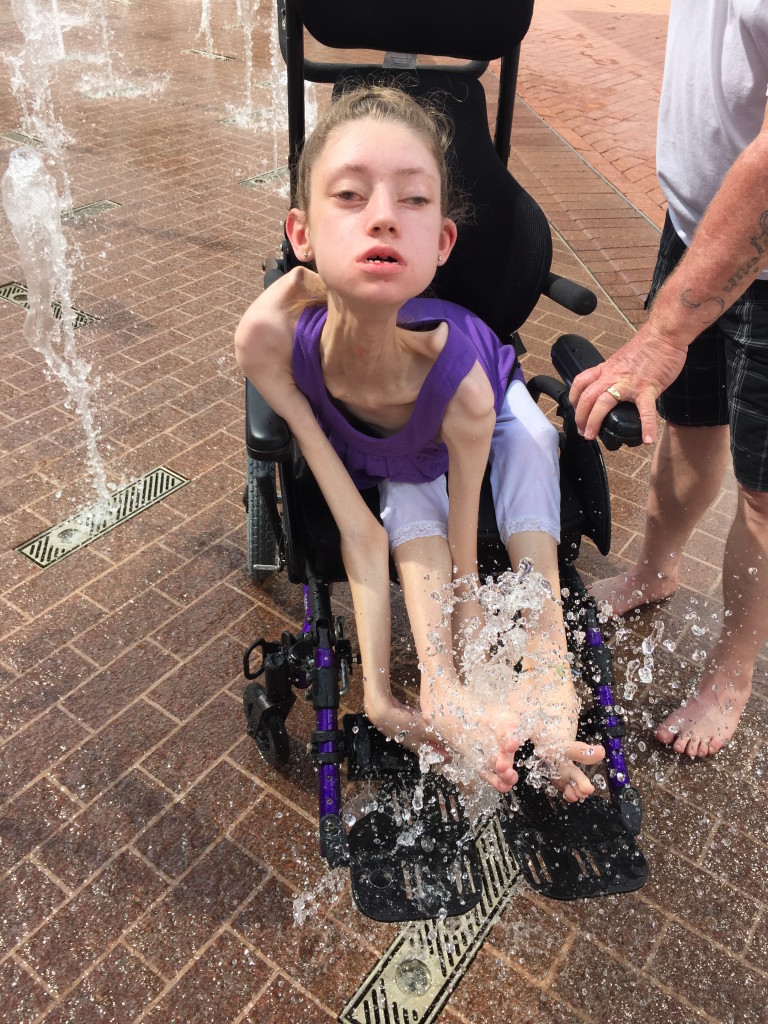
In this study, the median age of participants was 17.9 years at enrollment, with a shorter D4Z4 region (median of 16 kb) as expected for early-onset FSHD. While the data analyses are ongoing, we found that extramuscular features such as retinal disease and cognitive disability are relatively uncommon. One-quarter of the participants had a history of hearing loss, and 20 individuals (38 percent) are dependent on a wheelchair for part-time or full-time assisted ambulation.
Many individuals reported symptoms such as muscle pain and/or fatigue, and their pulmonary functions will need to be followed closely due to initial testing showing lower lung capacity.
Despite their prominent muscle weakness, many individuals compensated well and had a relatively good psychosocial quality of life.
In addition to evaluating clinical presentations, we studied the blood samples collected from participants by using a mass spectrometry-based proteome profiling technique to identify protein biomarkers in peripheral blood samples, which may potentially be used as surrogate markers for evaluation treatment efficacy in future clinical studies.
The research team recently received additional support from the FSHD Global Research Foundation and aTyr Pharma to conduct a longitudinal study with this patient cohort that includes three additional visits (six months apart). This will allow us to study changes over time in manual and quantitative muscle strength and function testing, as well as changes in the clinical phenotypes in children and adults with infantile-onset FSHD. The blood samples collected will allow us to better prioritize potential biomarkers.
This study would not be possible without the initial support from the FSH Society, followed by support from Muscular Dystrophy Canada and the FSHD Global Research Foundation. The international collaborative effort made it possible to identify and recruit participants all over the world.
Last and most importantly, this study would not be possible without the support from all the participants and their family members.
The valuable clinical and biological data generated from this study will greatly increase our understanding of early-onset FSHD. A well-characterized patient cohort is also an ideal population for future clinical trials.
Drs. Chen and Mah are investigators for the Cooperative International Neuromuscular Research Group (CINRG) studying early-onset FSHD. The FSH Society co-funded this study with the FSHD Global Research Foundation and Muscular Dystrophy Canada.


This is the first time I have seen pictures of young people who have been affected by this awful thing like my granddaughter. She was around 3 when it started. Shortly after FSDH was stated. Jessie is now 33 years old. It has been a very hard life but she never loses her optimistic positive attitude. We were told she wouldn’t live to be a teen. Thank God her parents didn’t accept that.