The cause of facioscapulohumeral muscular dystrophy (FSHD) is thought to center on DUX4, a gene that normally is silent in adult skeletal muscle. When DUX4 gets “expressed,” as happens in FSHD, it activates other destructive reactions in muscle cells and causes muscles to degenerate. Suppressing DUX4 is a logical strategy to treat FSHD, and a new study suggests that this may be achievable using “morpholino antisense oligomers,” a type of synthetic molecule that blocks specific DNA sequences.
Morpholinos are being tested in experimental treatments for other diseases, including spinal muscular atrophy, Duchenne muscular dystrophy, and myotonic dystrophy.
The new FSHD study was the result of a collaboration between academic scientists and Genzyme. The academic researchers are members of the NIH-funded Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center for FSHD and work at the University of Massachusetts Medical School in Worcester, as well as the Kennedy Krieger Institute and Johns Hopkins School of Medicine, both in Baltimore, Maryland.
FSHD results from a perfect storm of things that go awry in the genetic machinery that normally keeps DUX4 safely under lock and key. These include the loss of D4Z4 repeat units on a section near the tip of the long arm of chromosome 4, the presence of a “permissive” poly-A haplotype that allows the DUX4 gene to get transcribed into messenger RNA, and hypomethylation of the D4Z4 units. All of these conditions have to be present in order for FSHD to occur in an individual.
The researchers tested various morpholinos and found that one, code named FM10, which targets the poly-A site, was strikingly effective in knocking down DUX4 expression. They also looked at several genes that are activated by DUX4, such as ZSCAN4 and MBD3L5, and found that their levels were also very low, confirming that DUX4 activity had been suppressed.
In addition, the investigators checked to see that FM10 did not result in potentially detrimental “off target” effects on other genes.
These experiments were done in cells derived from 11 FSHD patients and six first-degree, genetically related, unaffected relatives, as well as in mice that had been engrafted with human FSHD muscle (see “Human Muscle Grows in Mice,” FSH Watch Spring 2014). The study acknowledges the FSH Society for assisting with recruiting volunteers to donate muscle biopsies used in the research.
Future work will address whether morpholinos can achieve DUX4 knockdown when given systemically and longer term to a whole animal. Stay tuned.
Reference
Chen JC, King OD, Zhang Y, Clayton NP, Spencer C, Wentworth BM, Emerson CP Jr, Wagner KR. Morpholino-mediated Knockdown of DUX4 Toward Facioscapulohumeral Muscular Dystrophy Therapeutics. Mol Ther. 2016 Jul 5. doi: 10.1038/mt.2016.111. PubMed.
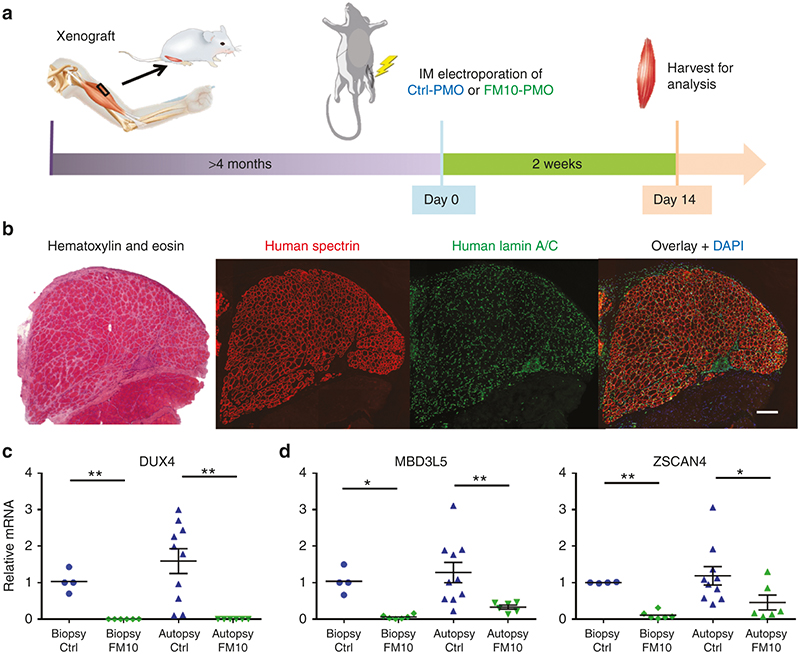
FIGURE CAPTION: FM10 knocks down DUX4-fl and DUX4 target genes in a human FSHD xenograft model. (a) FSHD patient muscle was transplanted into the hind limb of mice. The xenografts were treated with Control- or FM10- phosphorodiamidate morpholino oligonucleotides (PMO) and analyzed after 2 weeks. (b) The presence of human spectrin protein and nuclear envelope protein lamin A/C confirmed that the xenograft was well regenerated. (c) Significant decreases in expression levels of DUX4-fl and (d) of DUX4 target genes MBD3L5 and ZSCAN4 were seen in FSHD xenografts treated with FM10-PMOs, in contrast to controls. Ref. Chen JC, et al.
Image used under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.


This is such great news and so very encouraging to hear about university, clinic and biotech research work very involved in potential therapies for fshd. Very recently, the mid-Atantic fshd support group listened to a Washington DC area research doctor discuss her very similar antisense research work with a different biotech than that mentioned in this article. So, there are at least two biotechs out there pursuing antisense technology as a potential fshd therapy. I would assume with different compounds, but it is really great to see this kind of involvement and interest on the part of the biotechs and university and clinic researchers. Thank you all so much. Everyone, please continue to support the FSH Society so that they can expand on this incredible work.
This is the most positive thing I have read since I started researching FSHD after my 30 year old son was diagnosed last year. Being totally non medical and hopefully not sounding too desperate I have to ask how long would something as significant as this be expected to become a mainstream treatment.
Peter,
We’re all looking forward to that day when an approved FSHD treatment comes to market.
It’s a long path from a potential molecule to an Investigational New Drug (IND) to an approved and marketed therapy. A rough description of the process starts with a potential molecule, something that shows promise to treat a disease in a desired fashion. From there laboratory work is done ‘in vitro’ (in the glass), generally on cells outside a living organism.
If the molecule/drug shows promise ‘in vitro’, the pre-clinical process moves to ‘in vivo’ (in the living) and the molecule/drug is tested on living organisms, generally mice. That’s been a stumbling block in FSHD for a long time, because the disease process was not well understood and there were inadequate animal models. However, that’s changing much for the better with increased understanding of the FSHD disease process and advancements in animal models for FSHD research.
At this point, if the molecule/drug continues to look promising and can be proven safe, a biotech will be in the IND enabling state which is a process to prove to the FDA that the drug shows promise of effectiveness and is believed safe to administer to humans. Once the IND enabling work is complete, the biotech will submit an IND application to the FDA in hopes that the FDA will approve that the drug can begin trials in human subjects.
This is where clinical trials begin and they normally run in at least 3 phases.
Phase1 – usually a small number of subjects, quite often healthy individuals who do not suffer from the target disease and are usually compensated for their participation. The focus in Phase1 is safety.
Phase2 – A larger sample, but still reasonably small set of individuals that suffer from the target disease. The focus in Phase2 is safety and efficacy; is the drug providing therapeutic benefit.
Phase3 – An even larger sample of individuals that suffer from the target disease. The focus in Phase3 remains safety and efficacy.
Between these phases or running concurrently with these phases, there could be sub-phases such as a Phase1b depending on what that portion of the trial is attempting to prove.
If all is still going well after all phases of trial and the drug has continued to be proven safe and effective, the biotech can then apply to the FDA in order to market and distribute the drug as an approved therapy for the target disease. If the drug is approved, an ongoing PhaseIV step in the drug cycle continues. This is where doctors and patients could report to the FDA if they feel the drug has caused unwanted side effects or has proven therapeutic or not.
The time of the process can be 5-10 years. The process can be expedited somewhat for INDs that show exceptional results in trials, but safety and efficacy remain important in terms of the IND making it to market. It also has to be stated that many drugs never make it out of clinical trials.
For the biotechs that have made statements on progress on antisense therapy for FSHD, it looks like the two known biotechs are somewhere in the pre-clinical ‘in vivo’ stage to the IND enabling stage.
Thanks for taking the time to explain all that to me Jim it is much appreciated. Even though the timescales look a long way away I totally understand the need for the safety process to be carried out. Hopefully results will be so positive that we will be closer to the 5 year mark rather than the 10 year. Not sure if there is any similar research being carried out here in the UK but hopefully the medical research world will share their findings in the hope of some one somewhere producing an effective treatment. It would appear that unless a condition is deemed to be at epidemic proportions the pharmaceutical companies are not really interested in research and development of new drugs as there is no profit to be made. And I am sure I am not alone in being a parent who is distressed at seeing there once seemingly healthy child start to literally disappear in front of them. Made even harder when they themselves had to watch their mother die of cancer as there was no effective treatment for her type of cancer as it was ‘rare’ so little research had been undertaken for it! And now having just set out on the road to parenthood them self they are worried about how they will continue to support their family.
My fingers are crossed that the biotechs come good. Not sure how Clinical trails are done whether world wide or just within the USA but hopefully there will be an opportunity to allow my son to try and enroll. Once again thank you Jim.
You’re welcome, Peter.
FSHD research takes place throughout the world, not just in the US. There’s a lot going on, and no longer limited to academic labs, although we owe much to those research labs that were willing to look at FSHD before any commercial labs, and those academic labs continue to advance FSHD reasearch. There are a pretty encouraging number of biotechs working on FSHD therapies from myostatin inhibitors to increase muscle volume to antisense therapy to block the transcription of DUX4 to gene editing to “fix” FSHD at the DNA level to stem cell research. No one can say with any certainty that any of these attempts will make it to a marketable therapy, but the good news that didn’t exist just a few years ago is that there are a lot of biotechs trying. The more trying, the better chance exists that one or more of them will score a hit.
You’ll also notice in the Breaking News section of the FSH Society web site, the establishment of an FSHD Clinical Trial Research Network. That’s very encouraging and postive for several reasons. One, of course, is the desire to be ready when these biotechs get to the IND stage that we have a network of locations ready to take part in FSHD clinical trials. It also shows a great committment on the part of the FSHD community which biotechs will do well to take notice that there is an established network of locations with experts on FSHD ready for clinical trials.
You can watch for clinical trials at clinicaltrials.gov
My understanding is that all clinical trials taking place in the US must register the trial with clinicaltrials.gov, but you’ll see there are plenty of trials listed there with locations outside of the US.
You can also check clinicaltrialsregister.eu which I believe is the European equivalent of clinicaltrials.gov