by Peter Jones, PhD, and Takako Jones, PhD, University of Nevada, Reno
The aberrant expression of the DUX4 primate retrotransposon is the key mediator of all forms of FSHD. Thus, the DUX4-fl mRNA and protein are prime targets for therapeutic intervention.
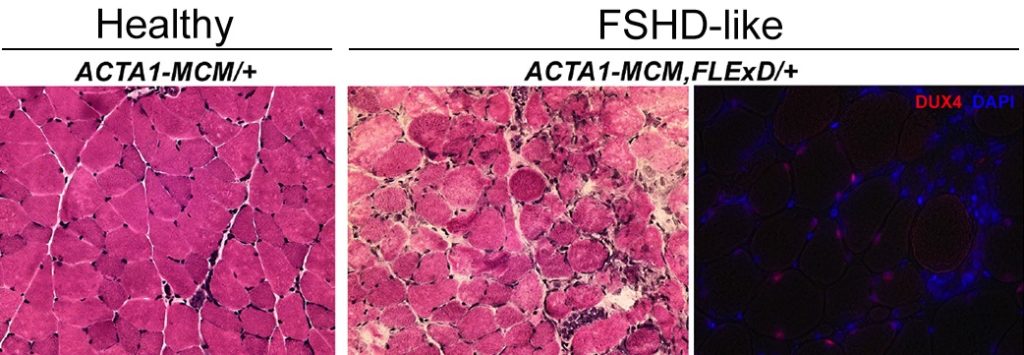
Our laboratory at the University of Nevada, Reno School of Medicine reports the successful generation and free distribution of a viable, fertile, and highly tunable phenotypic FSHD-like transgenic mouse model based on the controlled expression of DUX4. This mouse, referred to as FLExDUX4 (or FLExD), is now available from The Jackson Laboratory as B6(Cg)-Gt(ROSA)26Sortm1.1(DUX4*)/Plj/J, catalog #028710 (https://www.jax.org/strain/028710).
FLExD mice are available as males and females, hemizygous and homozygous, and will be ready for shipping after February 20, 2017. These mice are all very fertile, and have normal lifespans, and produce large litters.
DUX4 expression can be induced through expression of the Cre recombinase by a number of mechanisms but typically by mating with Cre-expressing lines of mice, resulting in an FSHD-like pathology.
The flexible design of this mouse model allows the investigator to induce DUX4 expression in either young or adult animals as well as control the degree of pathology and rate of disease progression, thus allowing for the assessment of prevention, inhibition, or reversal of pathology as desired, dependent upon the therapeutic intervention being tested. In addition, one can distinguish early, initiating events of pathology from cumulative, chronic pathological effects. The FLExD mouse can essentially be tailored to the type of therapeutic being tested or pathway being studied.
It is widely recognized that mouse-based preclinical studies have often produced inconsistent or even incorrect results due to lack of experimental rigor and/or lack of transparency in methodology, ultimately leading to poor reproducibility among labs and failures of clinical trials. The NIH has instituted a new policy demanding that investigators address the critical issues of rigor, transparency, and pertinent biological variables. We fully support this initiative and believe that a poorly characterized and inconsistent FSHD-like mouse model would only serve to set the field back, wasting time, money, and resources. Therefore, we have spent nearly two years characterizing the FLExD model and verifying aspects of its phenotype in collaboration with experts in several other labs.
Removing barriers, particularly the restrictions investigators place upon usage of lab-generated and patient-derived biomaterials, is critical to accelerating therapeutic development in FSHD. The ethical obligation to deposit cell and animal resources into publicly accessible biorepositories for unfettered access is critically important for the entire field. Thus, we have balanced our desire for a complete characterization and natural history study (which is ongoing) with the level of characterization required to make this model a usable tool for the FSHD field. Importantly, our model is highly consistent and reproducible within animals, between animals, and over many generations.
Therefore, in an effort to facilitate fast and clean transfer of animals and to relieve restrictions or complications imposed by investigator control of reagents and spur FSHD therapeutic development and preclinical testing, the currently unpublished FLExD line of inducible FSHD-like mice is now freely available from JAX labs.
Key elements of the FLExD model:
- Viable and fertile as FLExD/+ and FLExD/FLExD
- FLExD mice live a normal lifespan (>2yrs)
- Very low level of DUX4-fl mRNA and protein in the absence of Cre
- Viable and healthy double transgenic animals when mated with ACTA1-MerCreMer tamoxifen-inducible line
- Chronic low levels of DUX4-fl expressed throughout life with investigator-controlled bursts of DUX4-fl, recapitulating how we envision DUX4 expression in FSHD patients
- No adverse effects on transgene expression or inducibility through >6 generations
- DUX4-fl mRNA and protein expression is consistent throughout an animal’s musculature (contralateral muscles can be used as controls)
- FLExD x ACTA1-MCM cross produces large litters (10-12 pups) with many double transgenic animals
- Five severity levels of FSHD-like phenotypes have been analyzed
- Cre induction of DUX4 expression produces consistent pathophysiology from animal to animal
- Histopathology is consistent within each severity model
- Several other Cre expressing mouse lines of potential interest have been tested
Specific details for achieving the various severity models will be included in a publication sometime within the next year. Until that time, investigators can contact Peter Jones or Takako Jones at the University of Nevada, Reno School of Medicine, and we will be happy to help out.
This work was funded by the FSH Society (FSHS-22012-01), the Chris Carrino Foundation for FSHD, MDA (#383364), and NIH/NIAMS (R01AR070432, R21AR070438).


Leave a Reply