Written by Jim Albert
Eldersburg, Maryland
A cancer drug has been shown to potentially rescue some of the damaging effects of DUX4, the gene implicated in FSH muscular dystrophy. The laboratory of Peter Zammit, PhD, Randall Division of Cell and Molecular Biophysics, King’s College London, United Kingdom, in collaboration with Robert Knight, PhD, of the Department of Craniofacial Development and Stem Cell Biology at King’s, has published the results of its research on the activity of an FDA-approved drug, sunitinib, as having potential therapeutic activity for FSH muscular dystrophy (FSHD).
This research, sponsored by Muscular Dystrophy UK, the FSH Society, and the French
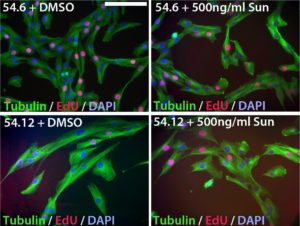
Myoblast (muscle precursor) cells from an FSHD “mosaic” patient (with a mix of normal and FSHD cells) were made into cell lines and labeled 54.12 (with FSHD genetics) and 54.6 (healthy control). Cells are labeled with tubulin protein (green) and DNA (red) to observe cell shape and cell division. Cell nuclei are blue. When sunitinib was added to healthy cells (top right), there was no effect compared to a control treatment with DMSO (top left). In contrast, 54.12 (FSHD) cells are long and thin when only DMSO is added (lower left), but become more similar to the 54.6 cells when sunitinib was added, suggesting that sunitinib is able to make the 54.12 cells appear more “normal.”
Muscular Dystrophy Association (AFM-Téléthon), arose from the finding that the gene RET (Rearranged During Transfection) was upregulated by the DUX4 protein in mouse muscle cells, as well as from work investigating the importance of RET in controlling the stem state of muscle progenitor cells in zebrafish.
While this research is at a very early stage and requires much further work, it is encouraging as it shows the potential for existing drugs to point to new strategies in the treatment of FSHD.
Sunitinib is approved for the treatment of certain types of cancer and inhibits cellular signaling by targeting several cell receptors, one of which is RET, a receptor tyrosine kinase. RET is activated by small proteins released by other cells, and until now has not been shown to be involved in adult muscle biology. This collaborative study revealed that RET is expressed in muscle stem cells and is required for their proliferation, indicating RET acts in the normal control of skeletal muscle formation and repair.
The overexpression of the DUX4 gene, or more properly DUX4-fl (full length), is widely believed to be the catalyst that sets off a chain of events leading to FSHD. While the research of the Zammit and Knight team showed that RET normally plays a beneficial role in muscle, in the presence of DUX4, RET production was increased, which can adversely affect muscle cell differentiation, the process of muscle stem cells forming skeletal muscle fibers. In tests performed in this research, sunitinib suppressed RET signaling to rescue DUX4-mediated inhibition of muscle differentiation in DUX4-expressing stem cells.
One observation made in this work revealed that RET is beneficial to healthy muscle formation, unless deregulated, when it is potentially detrimental. What might that mean in terms of attacking RET signaling as a therapeutic avenue in FSHD? Zammit answered this by commenting, “RET has a role in normal muscle formation in adults. Further research might focus on reducing levels from the abnormally high levels of RET signaling caused by DUX4 to more normal levels.”
The results of the Zammit and Knight team’s research in mouse cells was validated both in vitro and in vivo using muscle cells from an FSHD patient. The in vivo studies were performed by grafting the patient’s FSHD myoblasts into regenerating muscles of immunodeficient mice (lacking a normal immune system, so as to prevent immune rejection of the foreign cells).
Suppressing RET activity using sunitinib rescued the ability of both DUX4-expressing mouse myoblasts and FSHD patient-derived myoblasts to differentiate, or develop, into muscle. Sunitinib application also aided the engraftment and muscle cell differentiation of FSHD patient myoblasts that had been transplanted into mice.
This research showed that DUX4-mediated activation of RET contributed to the inhibition of myoblast differentiation and so might contribute to FSHD pathology by preventing stem cell-mediated repair of muscles. What’s more, the study showed that sunitinib rescued DUX4-induced pathology involving muscle cell differentiation, suggesting the therapeutic potential of tyrosine kinase inhibitors for the treatment of FSHD. (See figure.)
The researchers reported, however, that sunitinib does not prevent muscle cell apoptosis (“programmed cell death”), which may also play a role in damaging muscles in FSHD.
It is important to note that while research on RET signaling and FSHD is interesting, an increase in RET signaling is only one of the many consequences of DUX4 expression in muscle progenitor cells. Zammit commented, “RET is one of a plethora of changes that DUX4 causes in muscle. Countering RET effects leaves all these other changes in place, and they are still likely to be detrimental. Stopping DUX4 is probably the best option, as it stops all the downstream effects, too.”
While stopping DUX4 is indeed the leading contender for FSHD therapy, many doctors and scientists believe it may eventually be a cocktail of therapies which proves to be the best overall treatment of FSHD. This is especially true if one of the initial DUX4-targeted therapies only slows the expression of DUX4 rather than turning off DUX4 completely.
While it is encouraging that an already approved drug could possibly be part of such a treatment for FSHD, sunitinib does come with a variety of potentially serious side effects such that, while it may be a reasonable treatment for a severe form of cancer, the risks might not be appropriate for FSHD.
Zammit further commented, “While the data from this research is preliminary, uncovering the targets of sunitinib may identify targets that can be countered with safer drugs, or there might be a version of sunitinib appropriate for FSHD that has fewer side effects. Sunitinib, or another tyrosine kinase inhibitor, may not be a solo therapy but could be useful in combination with other drugs that target other aspects of the pathology, such as apoptosis.”
In response to questions about plans to expand on this research, Knight replied, “Our next step is to understand how sunitinib affects gene expression in FSHD patient-derived myoblasts. We have already started on this with the aim to identify candidate target molecules that we can focus on in more detail. In parallel, we intend to perform a screen for proteins showing altered activity in these cells in response to sunitinib, focusing on receptor tyrosine kinases.”
Reference
Louise A Moyle, Eric Blanc, Oihane Jaka, Johanna Prueller, Christopher RS Banerji, Francesco Saverio, Tedesco Stephen, DR Harridge, Robert D Knight, Peter S Zammit. Ret function in muscle stem cells points to tyrosine kinase inhibitor therapy for facioscapulohumeral muscular dystrophy. eLife. 2016;5:e11405. Online at: https://elifesciences.org/content/5/e11405.


Leave a Reply