The DUX4 gene in FSH muscular dystrophy is typically described as a rogue actor, a genetic oddball that is never supposed to be active in adult muscle, and is rendered harmless by an elaborate lockdown system. It is only when several parts of the “lock” mechanism break that DUX4 emerges to cause damage to muscles.
Yet researchers have also known that DUX4 is not simply a toxic accident, but is expressed in the male germline (sperm development) and embryo. What its role might be has become clearer. A quartet of papers has recently asserted that, far from being a bit of toxic genomic trash, DUX family of genes has a role at the very dawn of embryo development. (DUX4 is the human version; the mouse version goes by DUX; in this story we refer to them collectively as “DUX genes.”)
In the earliest stage of development, a fertilized egg divides into a cluster of cells that are featureless, quivering orbs of protoplasm. The genome in these undifferentiated cells is silent, like a symphony orchestra waiting for the maestro to pick up the baton. A process called zygotic genome activation (ZGA) causes the genome to switch on, and the orchestra to start playing. Inside the cells, networks of genes wake up and drive the development of cells down various pathways, leading to the formation of nerve, muscle, gut, skin—all the diverse cell types needed to build the tissues and organs of a fully functioning organism.
Research teams at the University of Washington, University of Utah, and Karolinska Institute, Stockholm Sweden (Hendrickson et al.; Whiddon et al.; Töhönen, et la.), reported that DUX turns on a set of genes that is expressed in two-cell (in mouse) or four-cell (in human) embryos. The third paper, by Didier Trono and his colleagues at École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, goes further, making the claim that DUX is the master switch—the Toscanini that wakes up the genomic orchestra, setting into motion the biological program that gives rise to a whole human being.
“We had good knowledge that DUX was doing some germline and stem cell programming, but the new papers really establish this,” said Stephen Tapscott, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington. He is a coauthor on two of the papers (Hendrickson et al. and Whiddon et al.). “In both situations, DUX is expressed at a time point that is important for setting up a permissive genome”—when many genes in the “orchestra” are allowed to make noise—“and that will give rise to an embryo.”
What DUX4 might be doing at the two- or four-cell stage is conferring “totipotency”—the power to become anything—which in molecular biology terms means “the ability to access all of your genes,” Tapscott explained. “We’ve known for a long time that many of the genes that DUX4 regulates are expressed in FSHD, and at these early time points.”
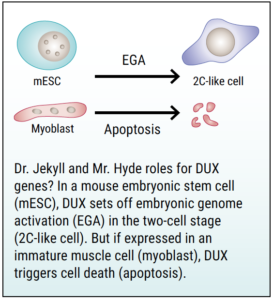 Why would turning on this early program be bad for adult muscle cells? Tapscott noted that the early-stage development of an embryo involves turning genes on and, just as importantly, turning them off when they are no longer needed. To go back to our orchestral analogy, the conductor not only rouses the violin section, but also hushes the trombones. Tapscott speculates that DUX4 may turn off the “skeletal muscle program,” thereby impairing the muscle’s ability to sustain and rejuvenate itself.
Why would turning on this early program be bad for adult muscle cells? Tapscott noted that the early-stage development of an embryo involves turning genes on and, just as importantly, turning them off when they are no longer needed. To go back to our orchestral analogy, the conductor not only rouses the violin section, but also hushes the trombones. Tapscott speculates that DUX4 may turn off the “skeletal muscle program,” thereby impairing the muscle’s ability to sustain and rejuvenate itself.
One of the surprising findings reported (in Whiddon et al.) is that mouse DUX has some significant differences from human DUX4. This is quite unexpected, because master genes in embryonic development are typically conserved with few changes from species to species. That’s because they are mission-critical, and any mutations in such a key gene would likely be an evolutionary dead end (that is, the embryo would die, and the mutation would not be passed on to future generations).
This insight could have important implications for developing a better mouse model of FSHD. “When you put human DUX4 into mouse cells, the mouse may not respond in the same way as it would to mouse DUX,” Tapscott said. “Using mouse DUX rather than human DUX4 might be better for modeling some aspects of DUX4 gene activity in the animal.”
In summary, these fundamental studies of the DUX gene family may yield at least two benefits to FSHD families who are waiting for a treatment. They suggest that DUX4’s role in early development could point to what goes awry in patients’ muscles—and how to reverse it. And this research could also contribute to building better FSHD mouse models, which are essential for studying any new therapies.
And there may be a third benefit. The discovery that DUX4 is not a genomic oddity but may play a central role in early development should attract attention from life scientists everywhere. That can only help speed up progress in understanding FSHD.
“It’s all fitting together. What’s nice is that pieces that seemed harder to connect are now getting connected more easily,” said Tapscott. “The pieces of the puzzle are starting to give us an idea of where things go wrong.”
References
Whiddon JL, Langford AT, Wong CJ, Zhong JW, Tapscott SJ. Conservation and innovation in the DUX4-family gene network. Nat Genet. 2017 Jun;49(6):935-940. doi: 10.1038/ng.3846. Epub 2017 May 1.
Hendrickson PG, Doráis JA, Grow EJ, Whiddon JL, Lim JW, Wike CL, Weaver BD, Pflueger C, Emery BR, Wilcox AL, Nix DA, Peterson CM, Tapscott SJ, Carrell DT, Cairns BR. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet. 2017 Jun;49(6):925-934. doi: 10.1038/ng.3844. Epub 2017 May 1.
De Iaco A, Planet E, Coluccio A, Verp S, Duc J, Trono D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat Genet. 2017 Jun;49(6):941-945. doi: 10.1038/ng.3858. Epub 2017 May 1.
Töhönen V, Katayama S, Vesterlund L, Sheikhi M, Antonsson L, Filippini-Cattaneo G, Jaconi M, Johnsson A, Linnarsson S, Hovatta O, Kere J. “Transcription activation of early human development suggests DUX4 as an embryonic regulator.” bioRxiv (2017): 123208.


What about the non muscular effects of FSHD ? Including learning problems and mental impairment. I didn’t read anything about this for a long time.
Is there any news about this ?
Thanks!!
FSHD has not been associated with cognitive impairments, but we have observed that some individuals, particularly those with early-onset FSHD, can sometimes manifest a variety of symptoms, including cognitive impairment, that may be due to the involvement of other genes. One can speculate that the genetic instability or de-repression of genes in the FSHD genetic locus might result in additional syndromes. This is a topic that needs further study.