The Spring edition of FSH Watch is here! Read on to catch up on all the happenings so far in 2018: This issue‘s highlights include: -Jim Chin elected Chair of… Read More »
The Watch has landed!
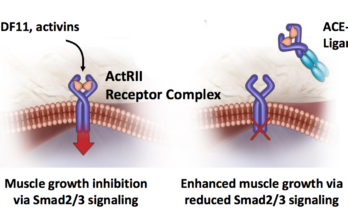
Acceleron Receives FDA Fast Track Designation for its FSHD drug
The Massachusetts-based biotech, Acceleron Pharma, issued a press release this morning with some encouraging news for FSH muscular dystrophy patients about its experimental drug, ACE-083 (see related story). Here is… Read More »
Moving toward well-being
Muscular dystrophy is a part of me, but it does not have me by DAVID YOUNGER, PhD Austin, Texas I was diagnosed with FSHD when I was about four, at… Read More »
ACE-083 Phase 2 trial results presented at AAN conference
Preliminary results from the ongoing phase 2 clinical trial of ACE-083 in FSHD patients were presented today at the American Academy of Neurology 70th Annual Meeting in Los Angeles, California.The… Read More »
Walking & Rolling Toward a Cure!
We are excited to announce the launch of our first nationally branded event, the Walk & Roll to Cure FSHD. This signature fundraising event will take place in September… Read More »