How DUX4 could cause FSHD without actually being there
BY AMANDA HILL, HIGHLANDS RANCH, COLORADO
In the FSHD research field, there is an emerging idea that the damage caused by DUX4 may linger on long after DUX4 is no longer present. But how can this be? To illustrate, imagine DUX4 as one of those trendy bath bombs that dissolve in your bath water ‒ drop in a bomb and it goes absolutely crazy! But only for a short time; soon the bomb dissolves and disappears. You wouldn’t know it was ever there, except that its earlier presence has now fundamentally changed the makeup of your bathwater, leaving behind a host of scents, salts, oils, fizz, and even colors.
DUX4 may act similarly in the muscle tissue of people with FSHD. It gets expressed, goes crazy doing all sorts of activities, then disappears. And it’s possible that what gets left behind is just as important as the initial presence of DUX4 in causing disease.
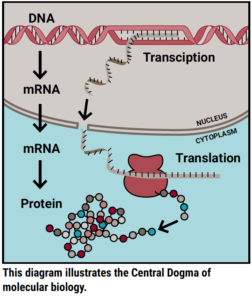 To explain how this works biologically, I first want to explain a molecular biology concept often referred to as the Central Dogma. The Central Dogma describes the most common flow of genetic information. Simply put, genetic information flows from DNA to RNA to protein. The first step employs a process called transcription to read a strand of DNA and synthesize a corresponding piece of RNA; the second step employs a process called translation to read that piece of RNA and build a corresponding protein. Proteins are the basic building blocks of all cells in the body and work together to perform biological processes.
To explain how this works biologically, I first want to explain a molecular biology concept often referred to as the Central Dogma. The Central Dogma describes the most common flow of genetic information. Simply put, genetic information flows from DNA to RNA to protein. The first step employs a process called transcription to read a strand of DNA and synthesize a corresponding piece of RNA; the second step employs a process called translation to read that piece of RNA and build a corresponding protein. Proteins are the basic building blocks of all cells in the body and work together to perform biological processes.
Molecular biologists trying to understand how DUX4 causes FSHD have largely focused their efforts on understanding how the first step of the Central Dogma is altered. FSHD is the result of genetic changes that allow DUX4 DNA to be erroneously available for transcription to RNA. Then, once made into a protein, DUX4 interacts with DNA to regulate transcription for many other genes and cause all sorts of disruptions that lead to FSHD. These disruptions happen within the first step of the Central Dogma. But what about the second step?
This question was recently addressed by Sujatha Jagannathan, PhD, then a postdoctoral fellow at the Fred Hutchinson Cancer Research Center, working with a team of scientists including Robert Bradley, PhD, and Stephen Tapscott, MD PhD.
Jagannathan explains the team’s thinking: “The field has long held the view that FSHD biology is all about the RNA molecules induced by DUX4. It made sense, because DUX4 is a transcription factor, whose main function is to induce the synthesis of hundreds of RNA molecules. However… recent results opened up the new possibility that DUX4’s effect on protein molecules might play an important role in FSHD. Therefore, systematically measuring the level of all protein molecules in DUX4-expressing cells was a key piece of the puzzle that we needed to understand FSHD pathogenesis, and that is what we set out to do in this project.”
Jagannathan used two different models of FSHD in which she had engineered normal muscle cells to express DUX4 protein. She then performed mass spectrometry to measure the levels of proteins, and at the same time, also sequenced all the RNA. With these new datasets, she was able to, for the first time ever, directly compare RNA levels and protein levels for thousands of unique genes in a model of FSHD.
One of the most important findings was that several genes that would normally respond to cellular stress had hugely elevated levels of RNA in the presence of DUX4, but their protein levels were only minimally changed.
Jagannathan explains, “We were surprised to find that many of the stress response genes that were transcriptionally upregulated when DUX4 is expressed (due to the stress it causes to cells) are not actually translated into proteins that can help the cells combat the stress. In other words, the cells are stressed but unable to cope with it. This could very well be one of the reasons that DUX4 is toxic to cells.”
Her analysis also validated a notable previous finding in the field: that DUX4 inhibits an important RNA quality-control pathway. Similar to the stress response example above, some genes that are required to maintain this quality-control pathway were being upregulated at the RNA level, but not translated into proteins that can actually do the needed work.
These findings are examples of post-transcriptional regulation ‒ biological processes that affect the second step of the Central Dogma ‒ and demonstrate why it is important for scientists to look at both steps to gain a better understanding of how DUX4 causes FSHD. As a result of this work, Jagannathan formed an important hypothesis that has the potential to change how we understand FSHD.
“Cells that are exposed to DUX4 proceed to undergo cell death even after they cease to express DUX4,” she said. “Our paper indicates at least two reasons for this: 1) the complete shutdown of RNA quality control can wreak havoc on the cells by generating faulty proteins, and 2) the inability to mount an effective stress response prevents the cells from coping with the loss in quality control and commits them to a path of inevitable cell death. Hence, therapeutic interventions that can reverse the downstream consequences of DUX4 expression should be an integral part of our strategy to combat FSHD.”
Jagannathan uses her team’s findings to propose two synergistic mechanisms that could contribute to the prolonged negative effects of DUX4. These are like the salts, oils, and fizz left behind by the bath bomb. She notes that effective therapies for FSHD may need to not only shutdown DUX4, but also reverse these lingering effects that could otherwise still lead to the death of muscle cells.
Jagannathan is now an assistant professor at the University of Colorado Anschutz Medical Campus and leads a team of scientists working to better understand how changes occurring at the second step of the Central Dogma contribute to the DUX4 “bath bomb.” In fact, earlier this year, postdoctoral fellow in Jagannathan’s lab, Michael Dyle, PhD, received funding from the FSHD Society to study the consequences of the reduced RNA quality-control pathway in FSHD.
You can learn more about Dr. Jagannathan’s lab and research at Jagannathan-lab.org.

REFERENCES
- Jagannathan S et al. Model systems of DUX4 expression recapitulate the transcriptional profile of FSHD cells. Human Molecular Genetics. 2016;25(20):4419-4431.
- Jagannathan S et al. Quantitative proteomics reveals key roles for post-transcriptional gene regulation in the molecular pathology of facioscapulohumeral muscular dystrophy. eLife. 2019;8:e41740.


“there is an emerging idea that the damage caused by DUX4 may linger on long after DUX4 is no longer present”.
Is it and idea on how long ? 1 year, 5 years, 10…….
Is it there any intuition about what may be “long after” ?
Thanks.