
Our community has been promised for decades that “one day in the future” there will be treatments for FSHD, and so it’s amazing to realize that day is almost upon us. The future is NOW for clinical trials. In 2019, we saw two Phase 2 trials—for Acceleron’s ACE-083 and Fulcrum’s ReDUX4 (losmapimod). A dozen biopharma companies and academic groups are working hard on the next wave of therapies, and we anticipate more clinical trials over the next few years. It’s very important that we use what we have learned so far to speed up the process and get treatments to our families as soon as possible!
Bottom line
We need to double the number of patients who are actively engaged, especially younger and moderately affected individuals.While companies are investing millions of dollars into research and trials, they can’t succeed without us. Our community must engage fully with the process. Across all research, 85 percent of trials face delays and 30 percent never even get off the ground because of the shortage of volunteers. This dramatically slows research, which means our families must wait longer for treatments.
This summer, Fulcrum Therapeutics launched its Phase 2 ReDUX4 trial of losmapimod, the first drug which has been shown to reduce the expression of a gene called DUX4 that causes FSHD. In the lead-up to this trial, Fulcrum conducted a “biomarker study” that is designed to evaluate the reliability of different measures of efficacy that will be used in the drug trial. We helped to recruit volunteers by sending emails to patients in the vicinity of the research centers involved in the study. We contacted nearly 700 patients and family members, and some 100 people responded. In the end, the study enrolled around 20 volunteers, just enough to complete the study, but it took several months longer than anticipated.
Why was it so difficult for this critical study to recruit enough patients? Researchers need to start with a well-defined population in order get a good signal from a study or trial. If patients are too diverse in their age, disease severity, etc., this can make the data difficult to interpret. For this reason, volunteers had to pass through many hoops to qualify. They had to be the right age. They had to be willing and able to complete multiple visits to the clinic over several months. They needed to be moderately affected—not too little, but also not too much. They had to have an actively affected leg muscle (identified by MRI.). They needed to be willing to have two muscle biopsies of that same muscle (one at the beginning and one at the end of the trial), because muscle tissue is the only method currently available to accurately measure DUX4 expression and the potential effect of losmapimod on the root cause of disease.
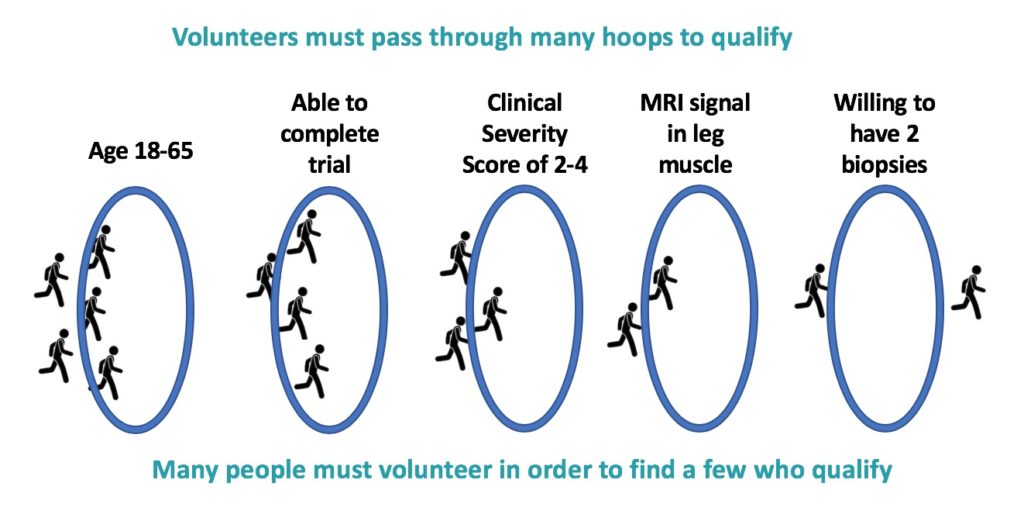
Given these complexities, it’s easy to understand why some volunteers did not meet the requirements for the study. The ReDUX4 clinical trial is now under way and must recruit 66 volunteers, who must all pass through the same stringent requirements. Every study and drug trial will face similar challenges. And this is why we need many more of you—patients and family members—to be actively engaged and join our “standing army” that is ready to leap into action.
The time is now. The need is urgent. Here’s what you can do:
- Join our contact registry for research;
- Make sure our emails to you aren’t going into junk mail folders;
- Read our e-newsletters and alerts to keep yourself up to date.
Learn how your contribution to our year-end campaign helps to speed up clinical trials.




Hi, I’m from Spain. I live in Palma de Mallorca. I am 33 years old. Currently, I’m slow and I have trouble climbing stairs but I climb them. If I serve you for something, count on me. I work as a nurse in the Oncology plant at the Son Espases University hospital. Thank you.
There will be trials of the Fulcrum drug in Valencia and Barcelona, Spain. We hope you’ll volunteer!
Until we get to the point of being able to measure DUX4 activity via blood samples, needle muscle biopsies are likely to be part of anti-DUX4 trials for at least the near future.
Patients shouldn’t let a needle muscle biopsy influence their participation in a trial or study without fully understanding a needle muscle biopsy.
A needle muscle biopsy is nothing like the open muscle biopsies many patients have undergone in the past.
A needle muscle biopsy is not nearly as invasive as an open muscle biopsy. With a needle muscle biopsy, the target area is shaved, numbed and a very tiny cut is made in the skin over the target muscle (a few millimeters). A thin needle is inserted one or more times to extract very small amounts of muscle which have been described as the size of a few grains of rice. The patient may feel a slight tugging or a small amount of discomfort, but generally no significant pain. Even in diseased muscle, this small amount of muscle is expected to regenerate.
Sterile dressing is applied to the area which can be removed in a day or two. In most patients, any pain is minimal and normal activity can resume in a day or two. In most cases, no lasting scar or mark remains after a month or two post biopsy.
If a needle muscle biopsy has been preventing you in participating in any studies, please talk to your study doctor to learn more about the procedure including all risks.
Hi Jim,
I live in the Northern Virginia(loudoun county) area outside of DC. I am interested in learning more about this clinical trial but like most hesitant with the biopsy of the muscle. In my 30’s, can walk but developed drop foot on my right side in recent years, now wearing AFOs. Been fully diagnosed with FSHD. Would you pls direct me to where I can go and call to learn more about the process in my area. I would greatly appreciate it.
Hi Teresa.
That is great that you are considering the Fulcrum trial. Yes, please learn more about a needle muscle biopsy and decide if that is an agreeable procedure for you and if your condition meets other trial requirements.
The clinicaltrials.gov web page for the trial is:
https://clinicaltrials.gov/ct2/show/NCT04003974?cond=fshd&draw=2&rank=1
Here are some study locations close to Loudon County.
The study coordinator at the Virginia Commonwealth University in Richmond is:
Nicholas Johnson, MD 804-828-9350 nicholas.johnson@vcuhealth.org
The study coordinator at Kennedy Krieger Institute in Baltimore is:
Genila Bibat, MD 443-923-2697 bibat@kennedykrieger.org
Wish there where more trials in th uk
ACE-083? Has not that been given up?
Development of ACE-083 has been halted, but the fact that a Phase 2 trial was done is an important achievement. While a clinical trial that does not prove the efficacy of a drug is often said to have “failed,” that is a misnomer. It is a successful trial if it resulted in a clear, actionable outcome (go or no-go). A failed trial is one that could not be completed (for example because it could not recruit enough volunteers, or the data were of poor quality and did not produce a clear conclusion).
I want to know why we need muscle biopsied when there are better ways to detect the effectiveness of the treatment. Invasive testing like this should be a last resort. Also, knowing how big the biopsy would be makes a huge difference. Many of us have little to no muscle left, a biopsy of 1% of a normal muscle could be 90% of ours.
Hi Joshua.
The primary outcome measurement of the Fulcrum trial is to detect change in DUX4 activity in muscle tissue with respect to dosing of the drug losmapimod. Currently, the most of accurate means of measuring DUX4 activity in muscle is via muscle biopsy. Scientists are working on ways to obtain similar measurements via blood markers, but we aren’t there, yet.
Please read my description of needle muscle biopsy a few posts up. If you aren’t familiar with needle muscle biopsy and only have open muscle biopsy experience, the two procedures are not the same. You can discuss this with your study doctor should you go forward in considering participation in the trial.
At this time, there is no other way to show that a drug has inhibited DUX4 expression in the muscle than to biopsy the muscle. Researchers are working to validate less-invasive tests, such a blood test, by collecting both biopsies and blood samples from patients and then seeing if the levels of certain molecules in the blood correlate with the changes in DUX4 expression seen in the biopsy. The biopsies needed for the Fulcrum trial are about the size of a grain of rice and can be done with a needle.
I live in Sydney. I am originally from Nepal. I am interested in learning more about this clinical trial. In my 30’s, can walk but developed drop foot on my both side in recent years, now wearing AFOs. Been fully diagnosed with FSHD
I am 73 years old. I live near Ft Myers FL. I have FSHD and so do my 2 sons. I do not walk unless I have something to hold onto but I primarily use my scooter. I can’t lift my arms I live alone in my home but count on my friends and neighbors. Gary, the oldest is 53 and can no longer walk and has trouble with his arms/hands. He lives in Traverse City MI He lives alone but has people come during the week and exercise him and will do anything he asks. My youngest son, Paul, is determined to keep moving! He still walks and is the most active of the 3 of us. He is married and has children and grandchildren. He is currently living in Sharon, SC
hi there, I living in Queensland in Australia and just wondering if there is any trial happening or will be in the near future?
Hola estoy espectante para el ensayo de losmapimod, cuando se abra en España, espero poder participar. Soy de Madrid y estoy diagnosticado de FSH.
I am the National General Manager for the Muscular Dystrophy Foundation of South Africa and is also affected by FSH. Unfortunately there is hardly any research opportunities in South Africa. Is there any way that we can get involved in your studies?
Hi Gerda, Thanks for reaching out. It would be helpful to know how many FSHD patients you have registered with the MDSA, your ability to contact them, and also the existence of clinical research centers that would have the interest and capability to run a study. This would provide a basis on which to bring clinical research and trials to South Africa. We do have a number of patients who have contacted the Society over the years and there’s a limited amount we can do from here without having some kind of infrastructure on the ground there. We’d be happy to discuss further. Please feel free to contact June Kinoshita at june.kinoshita@fshdsociety.org.
You hit the nail on the head with this post. It is time for clinical trials conducted with those who suffer FSHd. Thanks for such a persuasive post.
Active engagement from patients and families is crucial to overcome recruitment challenges in FSHD clinical trials. Joining registries, staying informed, and participating in research efforts can accelerate progress towards finding effective treatments.