The FSHD Society announced today that it has awarded $300,000 a year to expand the Facioscapulohumeral Muscular Dystrophy Clinical Trial Research Network (FSHD CTRN), with consideration of follow-on funding of $300,000 a year in 2021 and 2022. The network is a consortium of academic research centers in the United States and Europe with expertise in FSHD clinical research or in neuromuscular clinical trials. Jeffrey Statland, MD, University of Kansas Medical Center, and Rabi Tawil, MD, University of Rochester Medical Center, serve as co-directors of the network. The FSHD CTRN helps close gaps in trial readiness and also provides a network of sites with a centralized streamlined regulatory process, specific, common expertise in FSHD, and an engaged patient population ready to conduct efficient, high quality clinical trials.
Because of its prominent role in clinical trial readiness for FSHD, the CTRN is receiving FSHD Society funding to expand the existing consortium in the United States by four additional sites. The new sites are at the University Florida in Gainesville, University of Texas Southwestern Medical Center in Dallas, University of Colorado in Denver, and the Stanford University School of Medicine in Stanford, California. “This funding aims to ensure better coverage and access to patients,” said Jamshid Arjomand, PhD, chief science officer of the Society. “It also provides resources for the effective management and coordination across the entire network.”
The CTRN was formed to create an infrastructure of clinics with the expertise to accelerate therapeutic development for FSHD: by ensuring site training of key personnel; streamlining regulatory oversight and data capture; validating novel clinical outcome measures; and refining clinical trial strategies. Currently the network is running an NIH-funded study named Clinical Trial Readiness to Solve Barriers to Drug Development in FSHD (ReSolve), an 18- to 24-month observational study following 220 individuals with FSHD. The network was initially financed with seed funding from the FSHD Society, as well as other non-profits, and has since been awarded millions in additional funding from the Muscular Dystrophy Association and biotechnology companies that are pursuing therapies for FSHD.
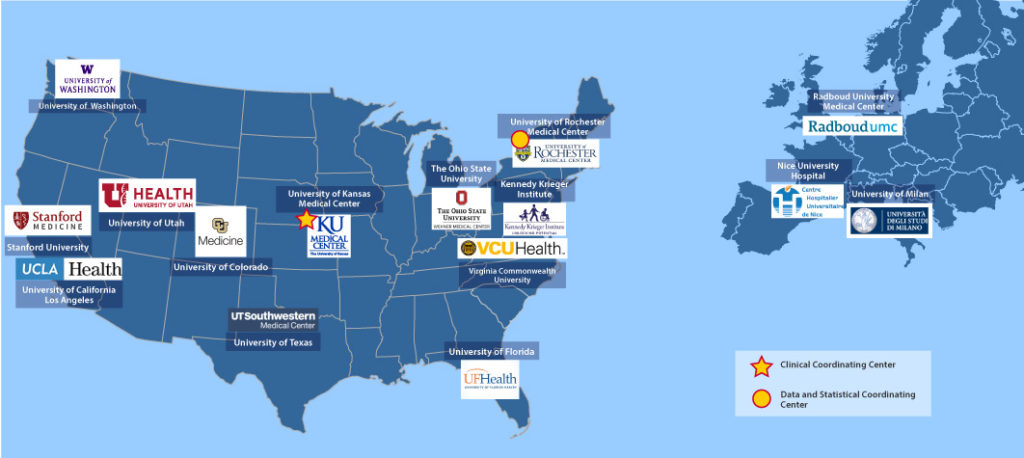
The current CTRN encompasses a total of 15 centers, with 12 sites in the United States and 3 collaborating sites in Europe. The central coordinating center is housed at the University of Kansas Medical Center, and the data and statistical coordinating center is at the University of Rochester Medical Center. “With the prospect of a growing number of FSHD clinical trials on the horizon, it will be critical for the CTRN to have the capacity and bandwidth to not only carry out the existing research studies but also successfully recruit and participate in clinical trials,” said Arjomand.
“We are grateful to the FSHD Society and like-minded organizations that have made it possible for the network to form and carry out studies to help bridge the gaps in clinical trial readiness,” said Jeffrey Statland, MD, associate professor of neurology at the University of Kansas Medical Center and co-director of the CTRN. “The additional sites will ensure that the CTRN will be able to meet the goal of trial preparedness and ensure well-trained FSHD clinical centers.”
About the CTRN
The Facioscapulohumeral Muscular Dystrophy Clinical Trial Research Network (FSHD CTRN) is a consortium of fifteen academic research centers (12 in the United States, and 3 in Europe) with expertise in FSHD clinical research or in conducting neuromuscular clinical trials. These centers leverage existing clinical trials infrastructure: including clinical investigators, research coordinators, study evaluators, and institutional resources like Clinical and Translational Science Research Awards (CTSAs) and local Research Institutes for clinical trial budget and contracting, and Clinical Research Centers, which provide subsidized clinical research space and facilities.


Great! Expanding the CTRN is the single most positive step we can take to engaging more patients in FSHD clinical studies.