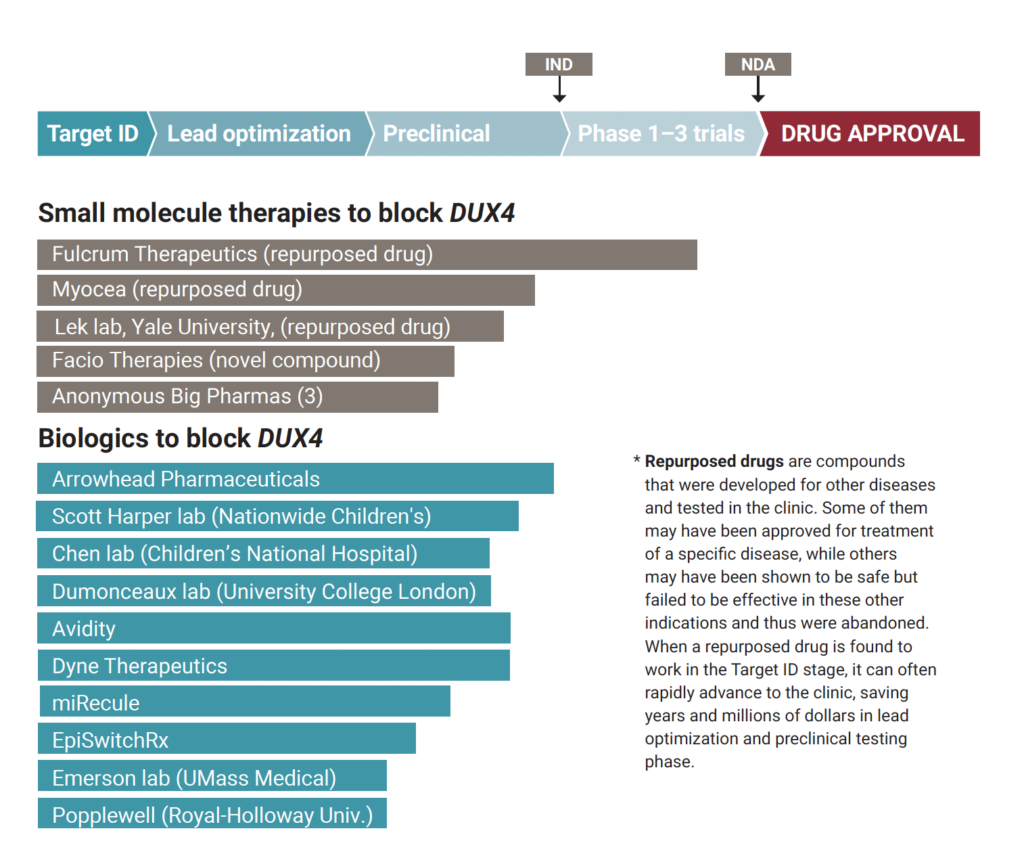
Growing numbers of companies and academic laboratories are pressing forward with early-stage drug development efforts. This chart shows how far various candidate anti-DUX4 drugs have progressed along the path to FDA approval. DUX4 is considered a key gene causing FSHD.
The process begins with finding a “target,” or biological disease mechanism, that can be modified by a therapy. Candidate compounds are identified based on their ability to “engage the target” and alter the disease mechanism (typically done in a test tube). Compounds that have the best properties to succeed as a drug are selected to be a “lead” (this is “lead optimization”). Lead compounds then go through a series of “preclinical” experiments, such as testing in an animal model. If a compound looks promising, a company may file an investigational new drug (IND) application with the FDA seeking permission to begin clinical trials in humans. The drug must then go through several trial phases before the company can file a new drug application (NDA), seeking permission from the FDA to put the drug on the market.
Small molecule drugs are chemical compounds that consist of up to about 100 atoms and are made through chemical synthesis. Being small, they can be more easily administered, for example, as a pill and pass through the digestive tract into the bloodstream to reach and penetrate cells. Biologics are very large molecules (such as RNA and proteins) derived from living organisms. They tend to be more precise and targeted, and therefore more effective. Because of their large size, they are usually delivered by injection or infusion, and getting them inside cells can be challenging.


Please hurry
Yes. ????
Thank you for the hard work. I think more can be done. Id like to raise money and awareness again for this cause. I don’t want to see fshd [ever] go to the wayside. It would be so incredibly amazing and we’d be so grateful if this could work and help people, and change and improve lives (mine as well). Thank you so much. ????
I have FSHD but I’m older (74). My 2 sons have it also. I just would love to have this to be a cure for them!!
Please get as many clinical trials as possible started. My mom was 62 when she died from complications from having FSHD and crappy family genetics in general. I’m in my 30’s and I have this condition. I would love to at the very least stop the progression. Prayers for more trials and a thank you for all that you do.
Would be nice to have a break through drug to treat my Muscular Dystrophy. I’m 43 this year and found out I had MD at age 13. Hopefully I can receive treatment in Australia where I live to help give back more independence to me that I’ve lost.
My husband is 69 with FSHD type 2 and 2 of our sons have the same. I agree with the above comments…please hurry with a cure/treatment/or just something to stop the progression. Thank you for all the work being done.
Thank you for this interesting summary !
I have read the article, but I would like to ask a clarifying question. Are there 12 different medicines being worked on right now? If one goes all the way to “drug approval”, can anyone with FSHD get it?
it is great to know where we r with our cure for fshd ? really if the world scientist and Govt wish to fight it out it would not have taken so long .please enroll my name in trails.
I’m from Poland . I’m 54 years old . I have been living with this terrible disease for 25 years. I feel lonely because I don’t know any person with this type of muscle atrophy. I’m also looking forward to the day when I find out that finally there will be this little, magic pill that will change my life.
My child is 12 years old now suffering from this disease, and I want to see him walk and run without falling to the ground, Lord, I hope everyone is cured
This is the first case we have in our large family, and we do not know the reason. We are all normal. Only this boy and there are not many cases in the Arab Gulf. May everyone be pleased with this wonderful news.
I have FSHD but I’m older (72). My 2 sons have it also. I just would love to have this to be a cure for them!!
Please hurry
Please, please hurry.
If they can push through the vaccine in a short time why can’t they do it for FSHD.
Do you know how difficult it is to which your child waste away in front of your eyes.
Please check out Arrowhead Pharmaceuticals press release dated today regarding their pre-clinical data for ARO-DUX4 to treat and reverse FHSD!
https://ir.arrowheadpharma.com/static-files/010912ca-0571-4009-962c-b0e2eae09e03
1. How many years is it going to take to get this treatment at the clinic? Every conceivable way to pare the time in which access is allowed/enabled to the severely affected should be explored and acted on.
2. All FSHD Therapy trials should be structured to enable/allow expanded access as early as during phase II. The severity of this disease demands it. The Fulcrum phase IIb results confirm DUX4 as an appropriate target in humans, so once safety is established in phase 1 there is no reason to not do expanded access during phase II trials. It is totally infuriating and unacceptable that this was not done in Fulcrum’s trial considering the collective loss of function those with FSHD suffered which could have been avoided had expanded access been allowed/enabled during phase II.