By June Kinoshita, Director of Research and Patient Engagement
“Positive benefit/risk supports losmapimod’s potential to be a transformative therapy for the treatment of FSHD”—Fulcrum Therapeutics
Fulcrum Therapeutics announced today that losmapimod, its experimental therapy for facioscapulohumeral muscular dystrophy, produced statistically significant improvements in function and decreased fatty infiltration of muscle in its ReDUX4 clinical trial. ReDUX4 was a randomized, double-blind, placebo-controlled Phase 2b clinical trial in 80 participants. The trial was conducted in multiple sites internationally and was designed to investigate the efficacy and safety of losmapimod taken in 15-mg pills twice per day. Based on today’s results, Fulcrum said it “plans to meet with health authorities, including the U.S. Food and Drug Administration (FDA), in the second half of 2021 to determine the regulatory path for losmapimod in FSHD.”
ReDUX4 looked at a variety of indicators of patient physiology and function, including muscle biopsies, magnetic resonance imaging (MRI), muscle strength and function, and patient questionnaires. Some of these measures included tools, such as MRI-informed biopsies to look for genes activated by DUX4 (the gene that causes FSHD), whole-body MRI, reachable workspace (RWS), and FSHD timed-up-and-go (FSHD TUG), which were developed in preparation for this trial. The COVID pandemic forced Fulcrum to extend its trial from 24 to 48 weeks—enabling the collection of additional data that allowed these positive outcomes to be more clearly demonstrated.
Other companies will be keenly interested in Fulcrum’s data because they indicate which outcome measures are the most robust for future FSHD clinical trials. They will also help trial researchers model the number of patients and duration of trial needed to demonstrate the effect of future drug candidates.
The entire FSHD community owes deep gratitude to the patients, caregivers, researchers, clinicians, funders, and advocacy groups that have brought us to this milestone.
Here is the recorded webinar with Fulcrum Therapeutics on June 24, 2021.
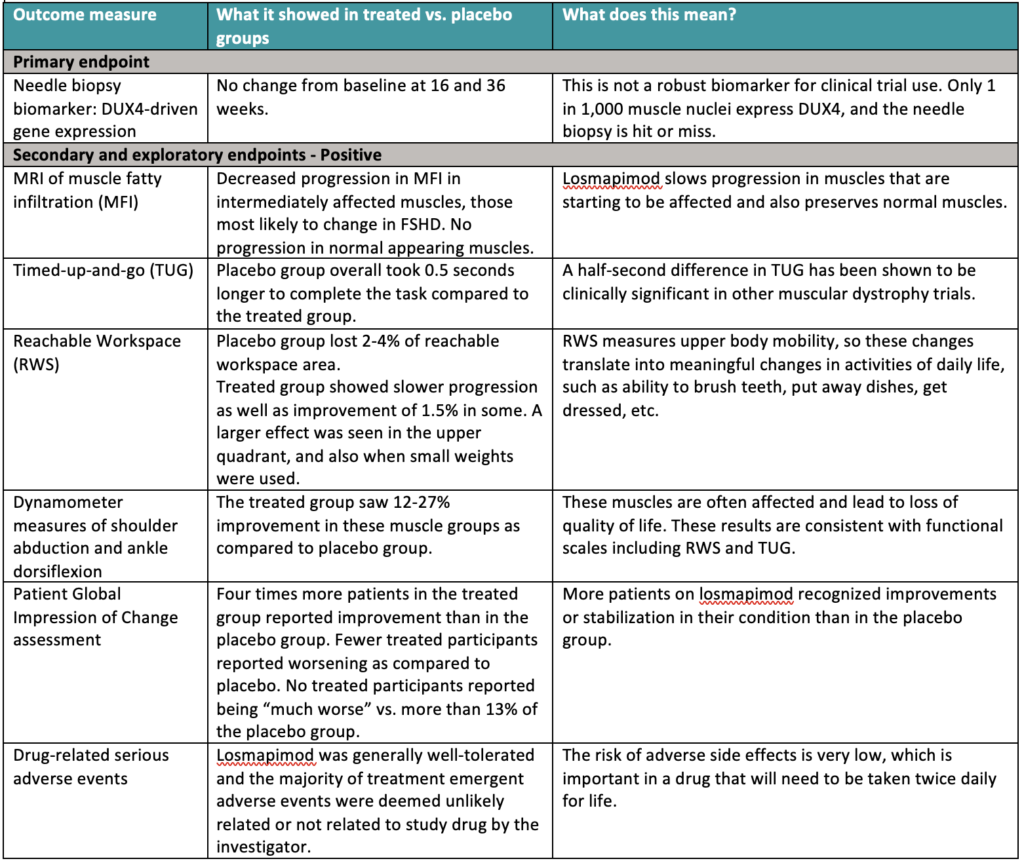
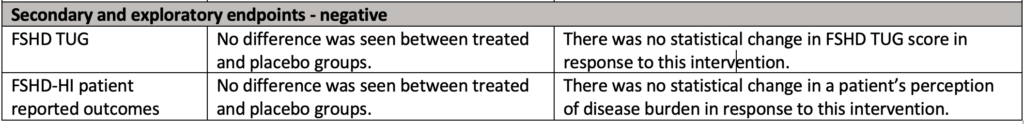
What did the data show?
There’s a lot to take in so we created this summary table:
What does this mean for future FSHD trials?
The primary endpoint—a change in DUX4-driven gene expression—was negative. Isn’t that bad? Not necessarily. It is disappointing, but hardly fatal. Analyzing muscle tissue from needle biopsies seemed promising in the laboratory. But in the actual trial, it proved to be challenging. DUX4 is expressed unpredictably and in only 1 in 1,000 muscle nuclei. It is the proverbial needle in a haystack. “We were pretty confident this drug would have effects on DUX4,” said Christopher Morabito, MD, chief medical officer at Fulcrum, but “were unable to see DUX4-driven gene expression due to the fact that it is stochastic and sparse.” Failing to meet the primary endpoint does not mean that the drug did not reduce DUX4 expression, only that the method used was not robust enough to show it.
The secondary endpoints were included in the trial to gather preliminary data on whether losmapimod might show some clinically meaningful benefit—which is what the FDA ultimately wants to see. Such endpoints were assumed to be too high a bar for a phase 2 trial. Ironically, it is these secondary endpoints that turned out to show a significant change. That’s what we mean when we say this trial exceeded expectations. In sports terms, we hoped to hit a single but ended up hitting a double or maybe even a triple.
Importantly, slowing or stopping progression, as these ReDUX4 data indicate, is the highest priority for patients, as determined from our Voice of the Patient Report. Fulcrum referenced the report in their presentation and said this consideration “was front and center” in their approach to designing the trial.
Judging from the FDA’s recent accelerated approval of Biogen’s Aduhelm treatment for Alzheimer disease, losmapimod has a very strong case in our opinion to receive accelerated approval as well. Aduhelm was approved on the basis of changes to brain amyloid (which is a controversial biomarker for AD) despite the lack of any functional improvement. In contrast, there is a strong scientific consensus on the central role of DUX4 in FSHD and on losmapimod’s ability to reduce DUX4 expression, and Fulcrum was able to show meaningful benefit to patients. And as we mentioned above, meaningful benefit is the gold standard for the FDA.
If losmapimod were to receive accelerated approval so that the drug can be marketed, the FDA would likely still require a post-market clinical trial to gather more robust data on the drug’s clinically meaningful benefits. It’s unlikely that the trial would require the collection of muscle biopsies, as biomarker data in a phase 3 trial would not carry weight with regulatory agencies.
What does this mean for people with FSHD?
There are still hoops to jump through before people will be able to get a prescription for losmapimod. Fulcrum needs to meet with regulators and determine its path forward. The FDA (and in Europe, the EMA) needs to review the data and make a decision on whether to grant an accelerated approval or require additional clinical trials before allowing losmapimod onto the market. The fact that losmapimod has been given a Fast Track designation is like getting TSA Pre-check to get through airport security screening faster.
Fulcrum is evaluating all populations, including children and individuals who were excluded from the ReDUX4 trial, and “working with regulators to find the best path forward to get losmapimod to patients as quickly as possible,” said Michelle Mellion, senior medical director at Fulcrum. Here's the company's expanded access policy, which explains what types of patients are eligible and the process for requesting expanded access.
“These results provide strong support that treatment with losmapimod has a meaningful clinical benefit in relevant measures of FSHD disease progression, despite the challenges of measuring DUX4,” said Rabi Tawil, MD, ReDUX4 principal investigator and professor of neurology at University of Rochester Medical Center. “I am enthusiastic about the potential for losmapimod to offer meaningful improvements in preserving muscle function and patient quality of life.”
Read Fulcrum’s press release for more details: Fulcrum Therapeutics Announces Results from ReDUX4 Trial with Losmapimod in Facioscapulohumeral Muscular Dystrophy (FSHD) Demonstrating Slowed Disease Progression and Improved Function
Disclaimer: Information provided by the FSHD Society does not imply an endorsement of any of the drugs, procedures, treatments, or products discussed. Please consult your own healthcare provider about any medical interventions.



Thank you very much indeed for this write-up. I saw Fulcrum’s announcement earlier and grasped that it was, overall, good news, but this lays it all out in an accessible way. One question: will other companies (eg Arrowhead with their TRIM platform) continue to press ahead with their research? Is the patient population big enough to warrant continuing to find alternative possible therapies? thank you!
Our expectation is that the promising Fulcrum will be encouraging to other companies that have their own therapeutic candidates. Fulcrum’s data addressed the biggest concern we’ve heard from companies–that it may not be possible to show clinical benefit in the timeframe of a clinical trial.
Thanks for the summary! In a perfect world scenario, what would the time-frame be for this to be available as treatment?
Very hard to say. Fulcrum hopes to have a meeting with the FDA by the end of this year. If (that’s a big “if”) the FDA grants an accelerated approval, the company would need to ramp up manufacturing, put distribution channels in place, determine pricing, etc. Not a trivial undertaking, but they’d have every incentive to move as quickly as humanly possible.
I attended the Zoom Webinar presented by Fulcrum to present the results of the Phase 2b Clinical Trial Results for Losmapimod on June 24th. Apparently the Webinar was recorded but I have not been able to find a link to the video. Could you please post the link.
Thanks for your interest. We can’t post the video yet because it contains unpublished data. As soon as the data are published, we will be able to share the video. We anticipate this will take several months, as the study has to be written up and submitted to a journal for peer review.
I find this confusing. Yes they missed the primary endpoint but I feel this is more a problem with the testing methodology because there are clear measurable benefits in reduced fat infiltration and strength improvements in two key msucles, as well as patient perception of improvement.
The question is, Fulcrum made no announcement of a phase 3 trial, instead they’ll be talking with the FDA. Does this mean they think there’s potentially enough data in the trial to explore approval and then do a further trial when it’s ‘in the wild’?
Given the fast track designation and orphan drug status, what kind of time line could we expect if all goes well?
You’re spot on in your understanding of the primary endpoint and secondary endpoints (clinical benefits). Fulcrum needs to meet with the FDA to determine whether an accelerated approval will be grants or whether the FDA will require further studies, and what kind of studies. With the Fast Track process, this will move more quickly than it would otherwise, but one still has to go through all the hoops!
Also, do we know when and where the full presentation is available for viewing?
Thanks
We can’t post the video yet because it contains unpublished data. As soon as the data are published, we will be able to share the video. We anticipate this will take several months, as the study has to be written up and submitted to a journal for peer review.
You can view the presentation on Fulcrum’s investor page https://ir.fulcrumtx.com/static-files/0a6f0ec1-def9-4350-b741-1cecd62f103d. There is also a video available under Past Events in which Fulcrum presents the results of ReDux4 to investors.
Really? It is still a matter of jumping through hoops to get access to a no risk therapy that has been proven to have a functional and physiological benefit? Why not allow expanded access to the most severely affected immediately? How much additional functional impairment that otherwise could have been avoided must those with FSHD endure while this “jumping through hoops” is going on? And at the same time, losmapimod is going straight to phase III for covid without a phase I or II.
FDA guidance on expanded access states that it be restricted to patients who would not otherwise be eligible for the phase 3 clinical trial. Because Fulcrum has not yet had its meetings with the FDA, which are needed to determine the scope and inclusion/exclusion criteria for phase 3, it doesn’t know which patients would be eligible for expanded access. We find it very encouraging that Fulcrum has posted its expanded access policy on Losmapimod and contracted with a company that will process expanded access requests. We empathize with you and how frustrating it is to have to wait further. But suppose Fulcrum opened its expanded access immediately to patients like you before knowing what the phase 3 trial will require, and then it turns out the FDA demands that the phase 3 trial include patients like you? What if by this time, so many patients have received expanded access that there are not enough for the phase 3 trial? The drug could fail to get FDA approval, which would be a tragedy for everyone.
Losmapimod for COVID is an entirely different story. The FDA did not require additional safety trials because the drug had been extensively tested in healthy volunteers. (Note, a safety trial was required for use in FSHD patients because there is a possibility that individuals with FSHD might react differently to the drug.) And in a global emergency where there are tens of thousands of people sick with COVID who are willing to volunteer for the trial, it makes sense to go straight to phase 3.
If they are ramping up production for a phase 3 covid, it would be hard to believe supply would be an issue. Fshd expanded access would be minuscule compared to covid phase 3. My understanding of fda guidance is that expanded access is allowed and in fact encouraged as early as during phase 2 trials for those severely affected, do not meet criteria for current trials and no alternative treatment exists. The idea that somehow those with fshd would react differently regarding safety than the 3500 who went through a phase 1 earlier with losmapimod makes no sense. Or that the chances that someone with covid having an unique safety reaction to losmapimod would be any less than someone with fshd makes no sense either. The difference is that there is much greater urgency with covid than fshd so no delays for a phase 1.
Excellent summary, June—thank you.
The biomarker findings suggest additional inquiry not relevant to marketing approval: Might Losmapimod not reduce DUX4 expression per se but instead downstream gene expression?; and, Might a different selection of biomarkers show change, whether by upregulation or downregulation? Both questions lead to an even more precise understanding of FSHD pathology.
The trial actually looked at the downstream genes. That is in our minds, evidence of DUX4 expression, but it’s a less direct one. Sorry we leapfrogged over this detail in the interests of trying to simplify the explanation. We believe the problem is more with the needle biopsy method of sampling muscle tissue. There’s too much chance involved in whether the needle will hit a spot with cells that are highly express DUX4 at that moment, and then trying to repeat it months later. You’d have to get lucky both times.
Dear June Kinoshita
I agree it’s a hopeful time for FSHD research. I do disagree with the framing of your analysis here, and recommend the community looks at the full results provided by Fulcrum.
We can’t claim ‘Losmapimod preserves muscles’ as you have written in the table, the results were not significant.
You frame the secondary endpoint results as hitting a double or triple and they were too high bar for the primary endpoint. I disagree, secondary endpoints do not have the same statistical authority as the primary endpoint, secondary endpoint results should only be used to help interpret the primary result of the trial or to provide info for future research.
Finally your push for accelerated approval seems very premature. 48 weeks of study of a drug like Losmapimod which patients may have to take for the rest of their lives, is a very short study. Given the results were hardly conclusive, it’s a must this study should have further trials to determine significant efficacy and safety.
For those severely affected, statistically significant reduction in impairment is significantly better than nothing. Likely this would not be for life as better therapies come on line in the years ahead. It is certainly rational to believe the theory that the problem may not be with Dux4 suppression but rather measuring Dux4 (primary biomarker) with needle biopsies because it exists in such a small fraction of muscle cells and it comes in bursts. Sure run more tests but make it available immediately via expanded access for those severely affected.
We agree, although “immediate” expanded access is challenging. We are advocating for earlier expanded access to patients who would never be eligible for a phase 3 trial. It’s a matter of coming to agreement on how to define this group.
You make a case against accelerated approval for Losmapimod. It will be interesting to see whether the FDA reviewers agree with you.
Re: the MRI results, Fulcrum states “Losmapimod-treated participants showed decreased progression in the treatment efficacy composite measure of muscle fat infiltration as measured in intermediate muscles, those most likely to change (p=0.01*). Normal appearing muscles appeared to be preserved in the losmapimod group versus placebo based on a post hoc analysis.” My recollection is that ~13% of the placebo group showed the development of fatty infiltration in muscles that appeared normal at the beginning of the trial, whereas none of the losmapimod group did.
Typically in a phase 2 of a disease like FSHD, a surrogate biomarker is selected as the primary endpoint because it’s assumed that clinical benefit would be difficult to show. However, in a phase 3, the FDA requires a demonstration of clinical benefit. So, to show clinical benefit in a phase 2 was a surprise, a great one, because it shows that it’s possible to reach that higher bar. The fact that the trial was doubled in length by COVID may have played a significant role. However, it is possible the FDA will only consider the primary outcome and not allow an accelerated approval. We feel this would be a grave mistake, given the weaknesses in the biomarker method, the small but statistically significant clinical benefit shown, the strong evidence that the drug is safe, and the urgent, unmet medical need of the FSHD community.
Are any controlled studies proposed looking at combining this medication with some type of physical therapy?
That would be a great idea and might happen once an effective therapy is on the market. We feel treatment plus exercise would be a reasonable protocol to consider once there are drugs available. We’ve proposed this idea to companies but it’s a hard sell because it might make it difficult to interpret how much of a contribution the drug makes vs exercise. On the other hand, one can imagine that there might be treatments that would have a negligible effect on their own, but which could have a significant benefit only when combined with exercise. The same challenges hold for any type of combination therapy.
Are there any ballpark estimates on the likely cost of this medication per year? $100k, $10k or $1k
We have no idea.
Does Fulcrum’s expanded access policy allow patients to apply who have no been accepted into their trial?
We’ll be asking about that.
This is wonderful news to hear! We in the FSHD community have been waiting for any kind of positive breakthrough, and this sounds like a big one.
Thank you June for your work and all in the FSHD Society. It is very much appreciated!
What time frame are we as individuals with FSHD looking at with the possibility of getting this as a prescription 1yr 2yrs or more?
Fulcrum would need to obtain FDA approval first. We hope there will be more clarity on what form that will take fairly soon…
What I’d like to know was whether the 80 participants had similar muscles affected by FSHD. I ask this because the 12 – 27 % improvement in the shoulder/ankle muscles is testing only (2) muscle groups in the entire body (those thought to be most commonly affected) From my experience, the wide variety of phenotypes in the FSHD affected community would necessitate measuring muscle strength in tests specifically tailored for each participant. As such, measuring how long it takes for a person to rise from a chair seems rather vague way to test functions that are so specific to individuals. Also, is there any indication of how much this drug will cost?
The participants were selected for the trial on the basis of having affected muscles in the anatomical regions that were amenable to being tested by the available instruments. Also, each individual’s score at the end of the trial is compared to their own baseline scores at the beginning of the trial. The study looked for whether individuals’ scores change over the trial,
Re: cost, we don’t know yet.
Thanks for that. Cheers, Clare
June,
Thank you for answering all of the questions. I know researchers have worked very hard on treatments and cures. We are all hopeful for a speedy approval. Is there anything specifically ‘we’ can do to accelerate that process?
Also, based on the results of the study, is there a discussion of what to improve in the drug for better results?
Hello
When is this drug available worldwide?
Are there natural supplements supplements to stop progress?
Thanks
Hi,
Is there a scenario where Losmapimod would be approved by the FDA and available for patients with COVID sooner? If so, would it be accessible for people without COVID but with FSHD?
The Losmapimod trial for COVID-19 was discontinued because it did not show efficacy against the virus. Therefore, losmapimod is not yet on the market and so it’s not available for “off label” use.
Hello! My concern is that Losmapimod is granted approval, and being an orphan drug, is then granted 7 years of market exclusivity for FSHD. In that time frame, other companies may develop much more promising/effective therapies (Arrowhead, Dyne, Avidity, Myocea, etc.). Does that mean we’d be “stuck” with Losmapimod for 7 years before other companies could market their products to the FSHD community? I think we should consider the consequences of this.
Good question. The market exclusivity applies only to Losmapimod. It would not prevent different therapies from being developed and approved.
Thank you! That’s good news to hear!
Hi I might need to get the covid vaccine possibly I would get Johnson and Johnson because it’s not mrna and it’s more like a traditional vaccine I know that it’s recommended for those with fshd to take the vaccine and from what I understand is that it won’t affect or get in the way of any future treatment or cure for fshd so I was just wondering how there’s a way to know that if they weren’t tested together and especially since the long term effects of the vaccine are unknown which in itself is scary it would be tragic if someone takes the vaccine and then a treatment or cure won’t work because of it
Some future FSHD therapies may use adeno-associated virus (AAV) as the vehicle to carry a gene therapy into muscle cells. One experimental vaccine for COVID-19 is based on AAV (it is not on the market), and the thought is that such a vaccine could cause the immune system to develop antibodies to AAV. If someone who had this vaccine were to then be given an AAV-based treatment for FSHD, the antibodies could neutralize it so that it wouldn’t be able to deliver the gene therapy into the cells. As mentioned, there is no such COVID vaccine approved for use yet (it is being explored by Harvard, if we recall). So this is a purely speculative scenario at this time.
How is AAV different from ADV? Why won’t the body make antibodies to AAV if given an ADV vaccine?
AAV is different from adenovirus. If you’re given a vaccine based on adenovirus, the body would make antibodies to adenovirus, but not for AAV. But as I mentioned, there is currently no COVID vaccine on the market that is based on adenovirus.
Bu losmopinod ilaç ı nezaman çıkacak
Sehr geehrte Damen und Herren wie lange müssen wir FSHD Kranke noch auf das Medikament warten es wäre dringend notwendig sonst verlieren wir immer mehr Kraft wann ist es endlich soweit wann können wir ein Rezept bekommen bitte geben Sie mir mal Bescheid vielen Dank mit freundlichen Grüßen Matthias Jackel