by June Kinoshita, FSHD Society
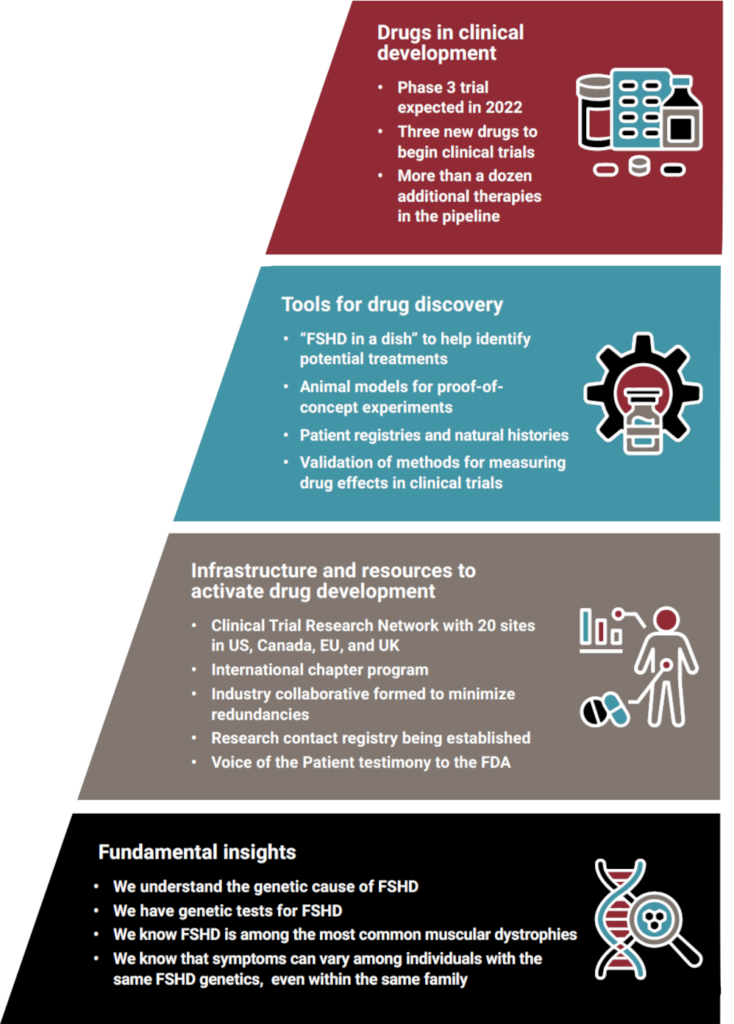 Over the next 12 to 24 months, four clinical trials for FSH muscular dystrophy are expected to launch. All of these upcoming trials all target the root cause of FSHD, the toxic DUX4 gene. For the first time, we have a chance at slowing disease progression, or even stopping FSHD in its tracks. This is why one of our urgent priorities is to make sure our community is trial-ready.
Over the next 12 to 24 months, four clinical trials for FSH muscular dystrophy are expected to launch. All of these upcoming trials all target the root cause of FSHD, the toxic DUX4 gene. For the first time, we have a chance at slowing disease progression, or even stopping FSHD in its tracks. This is why one of our urgent priorities is to make sure our community is trial-ready.
Since the founding of the FSHD Society more than 30 years ago, our journey has been a slow but steady climb up a steep hill. Now here we are, in 2022, with the summit in sight. Through funding of research grants, our Therapeutic Accelerator initiatives, and programs to expand and strengthen our community of stakeholders, the FSHD Society has had a disproportionately large impact. Take, for example, how the Society’s investment in the Clinical Trial Research Network has been multiplied many times over by other funders. As a result, we have greatly accelerated our ascent up that hill!
Our co-founders, Dan Perez and the late Steve Jacobsen, and the hundreds of early supporters who formed the FSHD Society, deserve our utmost gratitude. They knew the journey would be long and challenging, but they were driven by the conviction that they would deliver treatments and ultimately a cure if they – and we, their successors – persevered.
It’s worth taking a moment to reflect on where we stand today, thanks to the vision and tireless efforts of the FSHD community’s pioneers.


I never thought there would be treatment in my lifetime given the complexity of FSHD. While my focus has been on living the best life possible with FSHD and supporting others to do the same, I’m so grateful for your dedication and tireless efforts since you joined as well as Dan and all of the trustees (special shout out to Howard and much missed Carol), Mark, Beth and everyone before and since. It’s an exciting time and we wouldn’t be anywhere close to this without all of you. Thank you and keep up the amazing work!
I spoke with Steve Jacobsen several times in the early 90s and supported his efforts early on because at the time no existing effort had any chance of making anything happen that would be relevant to the time frame of my life. In fact, Steve Jacobsen told me that he started the society because he recognized that other “advocacy” entities at the time had no real focus on FSHD and had no chance of getting to a treatment in any meaningful time frame if at all. That being said, 12-24 months from now before starting a trial means probably at least 8 years (sometime around 2030 or later) before any of this means anything to those that suffer from FSHD. This is beyond an emergency situation for those that suffer from FSHD. And that time frame is far too long and completely unacceptable. FSHD Society should be doing much more in reducing these time frames especially for those most severely affected.
The 12-24 month timeframe is for all four of the currently planned clinical trials to begin enrolling patients. To clarify, one of these trials is a Phase 3 that will begin enrolling in the spring of this year. The FSHD Society’s entire program is aligned around accelerating therapeutic development.
My point is that if beginning trials start in 12-24 months from now, the way things are currently done, by the time recruiting, multiple trial phases, regulatory reviews and commercialization is done, it will be at least 8 years before any of these treatments could offer any relief to the general FSHD population. 8 years may even be greatly optimistic given that there is no real effort to streamline this process and most of these will be biologics. That time frame is simply unacceptable for a significant group of those that suffer from FSHD. This process of taking a technology from the lab to compassionate access (EAP) and commercialization needs to be turned on it’s head and become something that moves with real urgency. This is an absolute emergency and it is time that the process of moving technology from the lab to patients responds that way. This needs an approach closer to the Covid vaccine (lab to patient in months rather than years) rather than the normal (many years to decades) process normally used.
Another consideration is to use the new medications in a Compassionate Use way. The FDA allows certain medications to be used without full approval when there is an immediate life threatening illness. For some people with FDH, there life is immediately threatened by the disease. Losmapimod does not currently have this use.
I am from greece Thank you all of you who work so hurd for a tritment of FSHD. My wife has FSHD and i reading the news from the site Thank you again so mutch and also JUNE KINOSHITA take care.
I m from Romania . My wife have this disease and we need fast a treatment. Thank you!
I never heard of FSHD until my son was diagnosed. I believe a cure will be found. Now is the time of great discoveries in the field of genetics.
Already treated for Pompe disease and sinus atrophy.
I am happy to read all the news about discoveries in this area.
My heart wants to get treated soon, but I understand that the path is very long, just remember how many people have been looking for and launching a vaccine against polio.
With best wishes, Sergjj from Ukrine.