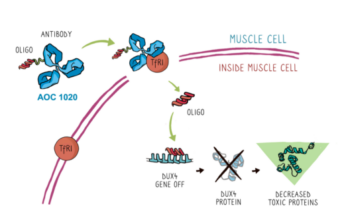
Trial has FDA approval. Start dates and locations have not yet been determined. Update as of 11/21/2024: AOC 1020 is now being referred to as Del-brax. San Diego-based Avidity Biosciences… Read More »
Avidity Biosciences announces Phase 1/2 trial for FSHD
Avidity receives IND clearance for FSHD therapeutic
AOC1020 will be the first RNA therapy to be tried in FSHD Avidity Biosciences announced today that it had received investigational new drug (IND) clearance from the U.S. Food and… Read More »
International research round-up
Report from the FSHD Society’s 29th annual International Research Congress by Alexandra Belayew, PhD, Mons, Belgium This year’s congress (June 16-17) opened with a keynote presentation by Lexi Pappas, who… Read More »
Abdominal and breathing exercises for FSHD
Disclaimer: Not every exercise is appropriate or safe for every individual, so please use your best judgement and consult your health care provider. We’re excited to launch Feeling Fit with… Read More »
A sense of belonging
by Raj Badiani, FSHD UK This year’s FSHD Connect was the first one that I’d ever attended, and for me this started late on the Friday evening. Sadly, I had… Read More »