Trial has FDA approval. Start dates and locations have not yet been determined.
Update as of 11/21/2024: AOC 1020 is now being referred to as Del-brax.
San Diego-based Avidity Biosciences announced this morning that it plans to launch a Phase 1/2 clinical trial of AOC 1020 in adults with FSH muscular dystrophy this year in 2022. The locations and start date for the trial have not yet been released. (For the latest trial updates, sign up to be contacted by the Society.)
Earlier this week, Avidity announced that the U.S. Food and Drug Administration (FDA) cleared the company’s investigational new drug (IND) applications of AOC 1020 for FSHD as well as of a second drug, AOC 1044, for the treatment of Duchenne muscular dystrophy (DMD) mutations amenable to exon 44 skipping (DMD44).
The FSHD trial is called FORTITUDETM and will help determine whether AOC 1020 is safe and well-tolerated in individuals with FSHD. The trial plans to enroll 68 adult volunteers with FSHD in a randomized, placebo-controlled, double-blind study. An advisory panel of patients and family members weighed in on the design and naming of the trial, the company said.
“Activity of AOC 1020 will be assessed using key biomarkers, including magnetic resonance imaging (MRI) measures of muscle volume and composition,” Avidity stated in its press release. “Though the Phase 1/2 trial is not statistically powered to assess functional benefit, it will explore the clinical activity of AOC 1020 including measures of mobility and muscle strength as well as patient reported outcomes and quality of life measures.”
While some of the FORTITUDE trial participants will be assigned at random to receive placebo, once the trial has been completed, all participants will have the option to enroll in an open-label extension study, the company said. In an open-label study, all participants will receive the active drug and will agree to continue to be monitored for long-term safety and outcomes.
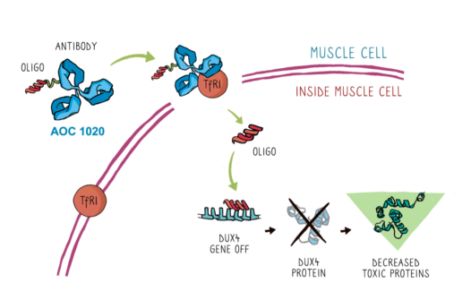
AOC 1020 is designed to treat the underlying cause of FSHD, the abnormal expression of a gene called DUX4. The expression of the DUX4 gene results in the production of DUX4 protein, which in turn triggers expression of other proteins that are thought to result in muscle weakness and damage in people with FSHD. AOC 1020 aims to prevent these toxic effects by interfering with the DUX4 messenger RNA and preventing DUX4 protein from forming. AOC 1020 consists of an antibody that binds to the transferrin receptor 1 (TfR1) conjugated with a “short interfering RNA” (siRNA) that prevents DUX4 mRNA from being expressed as protein. Data from laboratory studies showed that this approach prevented the development of muscle weakness in mouse models of FSHD, paving the way for the FDA’s green light to test AOC 1020 in human patients.
“We are grateful for companies like Avidity that are working to address a significant unmet need in the FSHD community,” said Mark A. Stone, chief executive officer at FSHD Society. “Patients as well as their caregivers and families live with the burden of this devastating disease every day and are in desperate need of treatment options that can improve quality of life. As FSHD is a progressive disease, the impact is debilitating and often results in an inability to do everyday activities like brushing teeth or getting dressed. There is a long road ahead, but today marks an important step and gives hope to everyone in our community impacted by FSHD.”
Video Webcast Information – Monday, October 3, 2022
The company is hosting Volume 4 of their virtual investor and analyst series on Monday, October 3, 2022 beginning at 10 a.m. ET / 7 a.m. PT to further discuss the AOC 1020 clinical development program. The event is a live video webcast and can be accessed here or from the “Events and Presentations” page in the “Investors” section of Avidity’s website. A replay of the webcast will be archived on Avidity’s website following the event.
Read the full Avidity Bioscience press release.



Good news