The foundations on which drug development happens
“Infrastructure is basic physical and organizational structures…needed for the operation of a society or enterprise.” —Oxford Dictionary
In a rare condition like FSHD, it’s unlikely for any one research center to be able to enroll enough patients for clinical studies or drug trials. Also, when patients and disease experts are few and far between, it’s difficult to have an impact. That’s why it has been a high priority to establish and support infrastructure—systems that enable patients, healthcare providers, and researchers to connect and work together, so they can set up standards and function as “centers without walls.” Just as good highways, reliable energy, and sound legal and financial systems are essential for driving economic growth, investing in research infrastructure greatly speeds up research and clinical trials.
Clinical Trial Research Network (CTRN)
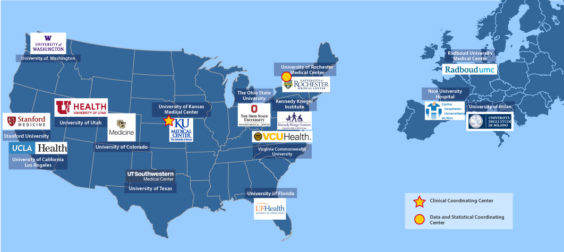
In 2016, the Society put seed funding into the FSHD Clinical Trial Research Network (CTRN), which has catalyzed clinical research. The CTRN is now running MOVE and MOVE+, the largest-ever natural history studies in FSHD, and is a cornerstone for multiple clinical trials.
We have continued to support the CTRN, which has expanded from the original 4 sites to 21+ sites, including six in the EU and UK, and three in Canada. CTRN co-director Jeffrey Statland even mentioned the possibility of future sites in Brazil and India at a recent meeting. We’re excited that there could be opportunities to engage patients in research in more diverse regions of the world. Not only will such sites bring help and hope to many more families, but we will undoubtedly gain valuable new insights into FSHD.
One thing we hoped the CTRN would do is attract significant additional funding into the FSHD space. That goal has been reached spectacularly, with millions of dollars from NIH, MDA, SOLVE FSHD and other donors, as well as contracts from pharmaceutical companies. This is a great example of how the Society leverages your investment to bring many times more dollars into the field.
“Soft” Infrastructure of care standards and health economics
In addition to the CTRN’s physical centers with trained researchers, we need “soft” infrastructure, such as standards and benchmarks. To this end, we organized the first ever accredited clinical training course on FSHD so clinicians, nurses, physical therapists and any health care professional that provides care to FSHD patients can get up to speed with FSHD and get the appropriate educational credits needed to maintain their licenses.
We are now working on updating the international care guideline, which will set better standards for how medical providers around the world diagnose and manage FSHD. The guideline will recommend a core set of clinical assessments which doctors everywhere should perform and record in patients’ medical records. This in turn means that data extracted in the future from medical records will be standardized and can be aggregated and analyzed to provide insights from a far broader and more diverse population of patients than just those who volunteer for research studies.
Last but hardly least, the Society is investing in studies of the health economics—investigating the full socioeconomic cost of living with FSHD. More than 300 households filled out our 125-question TrueCost of FSHD survey. Those of you who completed this huge task deserve medals! Our health economics initiative aims to establish a model for doing similar studies in other countries and provide hard data on the disease burden—data that insurance companies and policymakers will reference when the time comes to determine fair pricing for future therapies.
It is beyond exciting that we are at a milestone in our journey when we are no longer agonizing over whether there will ever be treatments but instead are working hard to ensure that patients will have access to the treatments that we know are coming. We could not have come this far without you!



Leave a Reply