Bringing two technologies together for a best-in-class therapy miRecule, Inc., a biotech based in Gaithersburg, Maryland, today announced a strategic collaboration and exclusive license agreement with pharmaceutical giant Sanofi to… Read More »
Sanofi and miRecule to partner on FSHD drug program
SOLVE FSHD Invests US $1 Million in miRecule
Funds aim to accelerate development of best-in-class antibody-RNA conjugate to treat facioscapulohumeral muscular dystrophy GAITHERSBURG, Maryland and VANCOUVER, British Columbia – September 29, 2022 (View original source.) – miRecule, Inc.,… Read More »
Avidity Biosciences announces Phase 1/2 trial for FSHD
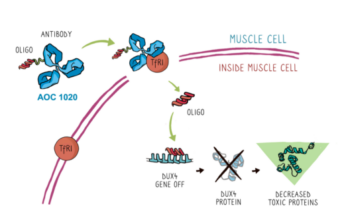
Trial has FDA approval. Start dates and locations have not yet been determined. Update as of 11/21/2024: AOC 1020 is now being referred to as Del-brax. San Diego-based Avidity Biosciences… Read More »
Avidity receives IND clearance for FSHD therapeutic
AOC1020 will be the first RNA therapy to be tried in FSHD Avidity Biosciences announced today that it had received investigational new drug (IND) clearance from the U.S. Food and… Read More »
International research round-up
Report from the FSHD Society’s 29th annual International Research Congress by Alexandra Belayew, PhD, Mons, Belgium This year’s congress (June 16-17) opened with a keynote presentation by Lexi Pappas, who… Read More »