University of Minnesota lab reports promising results in mouse FSHD studies
by Fred Thys, Weymouth, Massachusetts
Work by a team led by Dr. Rita Perlingeiro at the University of Minnesota suggests that FSHD patients could benefit from pluripotent stem cell therapy. “Pluripotent stem cells have the capacity to generate all the different cell types of the body,” said Perlingeiro, associate professor of medicine with the University of Minnesota’s Lillehei Heart Institute, in an FSHD Society webinar.
These cells can be reprogrammed from other cells in the body. “It sounds almost science fiction that you can reprogram your cells and they can become anything, and you actually can use cells that can generate all the cell types in the body,” said Perlingeiro.
She expects to file an Investigative New Drug Application with the Food and Drug Administration by this August for human trials for Duchenne muscular dystrophy. An Investigative New Drug Application is submitted for experimental treatments showing promise in clinical testing for serious or immediately life-threatening conditions while the clinical work and the FDA review take place. She envisions following up with limb girdle muscular dystrophy and FSHD.
Transplanting healthy cells helps in mouse models
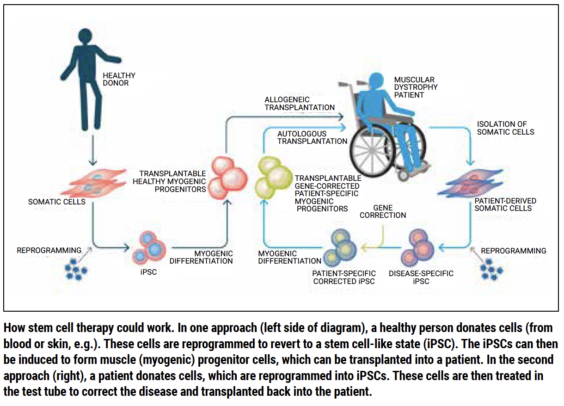 Perlingeiro’s research found that transplanting healthy cells into a mouse model of FSHD contributed to muscle regeneration. Her team found that implanted myofibers from a healthy donor seem to counteract DUX4-induced fibrosis and improve muscle strength over a short period of time. In the mouse model, the implanted myofibers seemed to be resistant to DUX4, and in fact the team found that bursts of DUX4- induced degeneration actually stimulate muscle regeneration in the implanted myofibers. Exposure of the engrafted cells to DUX4 makes them regenerate even more.
Perlingeiro’s research found that transplanting healthy cells into a mouse model of FSHD contributed to muscle regeneration. Her team found that implanted myofibers from a healthy donor seem to counteract DUX4-induced fibrosis and improve muscle strength over a short period of time. In the mouse model, the implanted myofibers seemed to be resistant to DUX4, and in fact the team found that bursts of DUX4- induced degeneration actually stimulate muscle regeneration in the implanted myofibers. Exposure of the engrafted cells to DUX4 makes them regenerate even more.
Perlingeiro visualizes two possible ways for the therapy to work. One approach would be to isolate cells from patients and reprogram them to become disease-specific induced pluripotent stem cells (iPSCs). The genetic mutation causing FSHD would then be corrected in the lab, myogenic progenitor cells would be generated, and they would be transplanted into the patient. “However, this approach would be really expensive,” Perlingeiro said.
Her team is proposing instead to start with another strategy: Generating cells from a healthy donor, reprogramming them to create iPSCs, generating healthy myogenic progenitor cells, and transplanting them into multiple patients. “We want to reach as many patients as possible,” Perlingeiro explained. In order to do that, Perlingeiro said, you would have to show that you can generate a consistent number of myogenic progenitor cells from pluripotent human stem cells. Her team showed 10 years ago that this is possible.
Human trials in Duchenne patients may pave the way for FSHD
Perlingeiro hopes that the first Duchenne patient would receive the first pluripotent stem cells, called myoPAXon, this year, for Phase 1 and 2 clinical trials evaluating safety and dosage escalation. Seven to 10 patients with Duchenne muscular dystrophy would be injected in the foot. As soon as her team can confirm that the cells are safe, the patients would be injected in the hand muscle.
Once the cells are proven safe for Duchenne muscular dystrophy patients, her team would be able to inject patients with limb girdle muscle dystrophy and FSHD in other muscles. Perlingeiro noted that immunosuppression would be required for injection, but she did not know for how long.
“Whether we can revert a muscle that’s really advanced, probably not, if it’s too advanced,” Perlingeiro clarified. “I’m not going to be promising to revert a muscle that’s gone. That would be almost like a miracle.”
Perlingeiro said she can envision that if a muscle is in advanced deterioration but “not gone,” it would be possible to see if the muscle can be made “healthier” through combined therapy with antifibrotics so that it can receive the cells. Her team has just received a grant from the National Institutes of Health to study this possibility.
One goal, she stated, is to understand if eventually, the cells can be transmitted through infusion into the bloodstream. Her team would prefer intra-arterial infusion to intravenous infusion to prevent the risk of cells being trapped in the lungs. With an intra-arterial injection, several muscles could be targeted at once. “That’s what we are gearing up for,” she added.


Do muscles affected by FSHD completely disappear at some point? I remember reading somewhere that there was some muscle mass left. “I’m not going to be promising to revert a muscle that’s gone. That would be almost like a miracle.” Is this statement directed at people with Duchenne, FSHD, or both?
It’s thought that at some point, muscle tissue is lost and replaced by scar and fatty tissue. However, it’s thought that there is an intermediate stage in which muscles are still hanging on. Dr. Perlingeiro is suggesting that if there is some muscle tissue left, the stem cells can integrate into it and build replacement muscle fibers. But if the “scaffolding” for muscle is gone, it might be impossible for the transplanted stem cells to integrate correctly.
I have FSHD. The first threshold is muscle mass dropping below a level where you can’t move the limb. From then on, the muscle wastes due to non-use + atrophy. I don’t know if it goes to complete zero or not. But it will look like all bone. I have that in my arms for example.
Have Masqular dystrophy but no deletion telling what should I do
Please help me
You must go to get one genetic test to found out which will be the most appropriate treatment for you, go to see your doctor and ask him, also you can seek help from a parent association depending were you leave, for example in US you have MDA
https://www.mda.org/care/mda-care-centers
Hope this help
Best Wishes
Michel