by Amanda Hill, Denver, Colorado FSHD clinical researchers recently published what may soon become a standard battery of assessments for use in clinical trials, an exciting and essential milestone for… Read More »
A new tool for measuring disease burden in FSH muscular dystrophy
The Watch has landed!
The Spring edition of FSH Watch is here! Read on to catch up on all the happenings so far in 2018: This issue‘s highlights include: -Jim Chin elected Chair of… Read More »
Acceleron Receives FDA Fast Track Designation for its FSHD drug
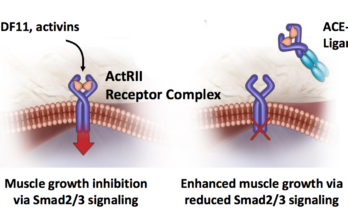
The Massachusetts-based biotech, Acceleron Pharma, issued a press release this morning with some encouraging news for FSH muscular dystrophy patients about its experimental drug, ACE-083 (see related story). Here is… Read More »
Moving toward well-being
Muscular dystrophy is a part of me, but it does not have me by DAVID YOUNGER, PhD Austin, Texas I was diagnosed with FSHD when I was about four, at… Read More »
ACE-083 Phase 2 trial results presented at AAN conference
Preliminary results from the ongoing phase 2 clinical trial of ACE-083 in FSHD patients were presented today at the American Academy of Neurology 70th Annual Meeting in Los Angeles, California.The… Read More »