The foundations on which drug development happens “Infrastructure is basic physical and organizational structures…needed for the operation of a society or enterprise.” —Oxford Dictionary In a rare condition like FSHD, it’s… Read More »
$3.4 million to speed us on our journey (Part 3)
$3.4 million to speed us on our journey (Part 2)
Building tools to accelerate drug development and clinical trials In the pioneering days of the Society, all our research funding went to basic research aimed at discovering the genetic cause… Read More »
$3.4 million to speed us along our journey (Part 1)
How projects we have funded will help us get more and better treatments to our community by June Kinoshita, FSHD Society Many of you have heard us describe the FSHD… Read More »
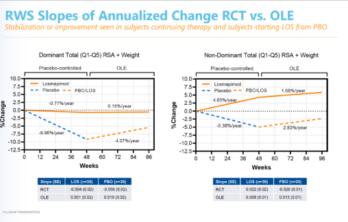
Losmapimod continues to show promise
Steady improvement seen in data from open-label extension study Losmapimod, a drug that is currently in a Phase 3 clinical trial for FSH muscular dystrophy, continues to slow or stop… Read More »
Epic Bio takes aim at DUX4
Updated 12-12-24 to reflect the chance in name from Epic Bio to Epicrispr Biotechnologies. Leading-edge CRISPR biotech chooses FSHD as its first disease target Epicrispr Biotechnologies, based in the San… Read More »