by Lawrence J. Hayward, MD PhD, University of Massachusetts Medical School To develop robust therapies for FSHD, we need a better understanding of the sequence of events occurring at the… Read More »
Dynamics of DUX4 downstream networks
FDA grants fast track designation to Losmapimod
From Global Newswire. Read full release. CAMBRIDGE, Mass., May 12, 2021 (GLOBE NEWSWIRE) — Fulcrum Therapeutics, Inc. (Nasdaq: FULC), a clinical-stage biopharmaceutical company focused on improving the lives of patients with genetically… Read More »
A researcher’s journey into FSHD
In this FSHD University Webinar from April 15, 2021, Angela Lek, PhD, a research scientist at Yale University School of Medicine, speaks about how her husband’s diagnosis with limb-girdle… Read More »
Arrowhead Pharmaceuticals announces FSHD drug candidate
Arrowhead Pharmaceuticals, Inc., a Pasadena, California, company, today announced ARO-DUX4 as Arrowhead’s first muscle targeted investigational RNAi therapeutic candidate. Using the company’s proprietary Targeted RNAi Molecule (TRIMTM) platform, ARO-DUX4 is… Read More »
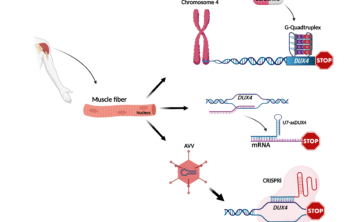
A trio of promising advances toward treating FSHD
Commentary by Emanuele Mocciaro, PhD, San Raffaele Scientific Institute, Milan, Italy Facioscapulohumeral muscular dystrophy (FSHD) is caused by the “anomalous” reactivation of the DUX4 gene. DUX4 has an important role… Read More »