From BusinessWire CAMBRIDGE, Mass.–(BUSINESS WIRE)–Acceleron Pharma Inc. (NASDAQ:XLRN), a leading biopharmaceutical company in the discovery and development of TGF-beta therapeutics to treat serious and rare diseases, today announced that the… Read More »
Acceleron Receives FDA Orphan Drug Designation for ACE-083
FDA orphan drug designation for Genea Biocells’ FSHD drug candidate
Genea Biocells, a San Diego-based biotech company focused on drug development to treat neuromuscular diseases, today announced that it has been granted Orphan Drug Designation by the U.S. Food and… Read More »
DUX may be a master switch of the genome
The DUX4 gene in FSH muscular dystrophy is typically described as a rogue actor, a genetic oddball that is never supposed to be active in adult muscle, and is rendered… Read More »
Rochester FSHD Family Day videos
Watch the videos here. The University of Rochester Fields Center for FSHD Research has posted a summary and videos from the 6th annual FSHD Family Day Conference held on April… Read More »
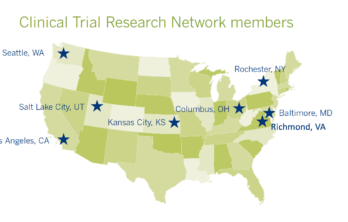
Clinical trial readiness study calls for volunteers
by Jim Albert, Eldersburg, MD The FSHD Clinical Trial Research Network (CTRN) is currently recruiting up to 160 FSHD patients across the seven CTRN sites to participate in a study called… Read More »