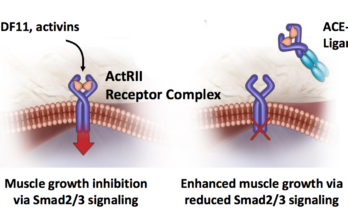
by Amanda Hill, Denver, Colorado FSHD clinical researchers recently published what may soon become a standard battery of assessments for use in clinical trials, an exciting and essential milestone for… Read More »
A new tool for measuring disease burden in FSH muscular dystrophy
Acceleron Receives FDA Fast Track Designation for its FSHD drug
The Massachusetts-based biotech, Acceleron Pharma, issued a press release this morning with some encouraging news for FSH muscular dystrophy patients about its experimental drug, ACE-083 (see related story). Here is… Read More »
ACE-083 Phase 2 trial results presented at AAN conference
Preliminary results from the ongoing phase 2 clinical trial of ACE-083 in FSHD patients were presented today at the American Academy of Neurology 70th Annual Meeting in Los Angeles, California.The… Read More »
Intelligent orthotics step forward at the MIT Media Lab
During the 2014 biennial FSHD patient conference in Boston, the FSH Society arranged for a group to visit the MIT Media Laboratory, where Professor Hugh Herr leads a visionary team… Read More »
How the FSH Society drives FSHD therapy development
This infographic from our FY2017 Donor Report illustrates how the FSH Society is involved at every stage of the treatment development process. We invest strategically at key stages along the… Read More »