Fulcrum Therapeutics has announced successful completion of patient enrollment in REACH Phase 3 trial The FSHD Society is thrilled to announce a significant milestone in the battle against FSHD. Fulcrum… Read More »
The FSHD Society Celebrates Fulcrum’s Milestone in the Fight Against FSHD
FSHD Lab Day was empowering for all
Uniting the FSHD Community in Advancing Research by Nizar Saad, PhD, Columbus, Ohio On July 8th, our lab at Nationwide Children’s Hospital (NCH) in Columbus, Ohio, in collaboration with NCH,… Read More »
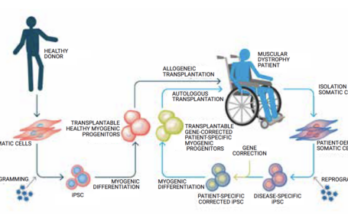
Making strides toward stem cell therapies
University of Minnesota lab reports promising results in mouse FSHD studies by Fred Thys, Weymouth, Massachusetts Work by a team led by Dr. Rita Perlingeiro at the University of Minnesota… Read More »
Arrowhead files for regulatory clearance to start a Phase 1/2 study in FSHD
From Arrowhead Pharmaceuticals PASADENA, Calif.–(BUSINESS WIRE)–Jul. 17, 2023– Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) today announced that it has filed an application for clearance to initiate a Phase 1/2 clinical trial… Read More »
Passion and hope at the IRC
The immeasurable value of face to face meetings by Raj Badiani, FSHD UK I was given the opportunity to attend the FSHD Society’s International Research Congress (IRC) in Milan this… Read More »