To crack the code of FSHD, patients are absolutely essential
All of the breakthroughs—the discovery of the genetic causes, understanding why some patients vary so greatly in the severity of their symptoms, teasing out the biochemical pathways that could point to future treatments—were made because patients stepped up to the plate.
Too often, we hear patients say they’ll volunteer when there’s a treatment. But we will never get to a treatment unless patients participate in fundamental research now. FSHD is uniquely human, so no laboratory mouse can ever fully model the disease. The genetic “package” that causes FSHD is found only in people. We owe an enormous debt to the patients who give DNA samples. Who submit to long interviews and exhausting physical tests. Allow a surgeon to cut out a small muscle sample. Who fight claustrophobia to lie in the narrow bore of an MRI machine.
Equally important are patients’ family members, both affected and unaffected, who provide the best experimental controls because of their shared genetic and environmental backgrounds. A parent or sibling who has very mild symptoms may hold the key to understanding the factors that protect against the full-blown development of FSHD symptoms in a more severely affected family member.
We are more hopeful today than ever before that a treatment is within sight. We cannot guarantee when that treatment will arrive, but here’s one thing we guarantee: If you volunteer for research, your participation will without question help move us a step closer to that day.
Scientific Overview of FSHD
Read the latest on wikipedia
Glossary of Scientific Terms
SOLVE FSHD Invests US $1 Million in miRecule
Funds aim to accelerate development of best-in-class antibody-RNA conjugate to treat facioscapulohumeral muscular dystrophy GAITHERSBURG, Maryland and VANCOUVER, British Columbia – September 29, 2022 (View original source.) – miRecule, Inc.,… Read More »
Avidity Biosciences announces Phase 1/2 trial for FSHD
Trial has FDA approval. Start dates and locations have not yet been determined. Update as of 11/21/2024: AOC 1020 is now being referred to as Del-brax. San Diego-based Avidity Biosciences… Read More »
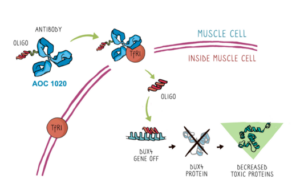
Avidity receives IND clearance for FSHD therapeutic
AOC1020 will be the first RNA therapy to be tried in FSHD Avidity Biosciences announced today that it had received investigational new drug (IND) clearance from the U.S. Food and… Read More »
International research round-up
Report from the FSHD Society’s 29th annual International Research Congress by Alexandra Belayew, PhD, Mons, Belgium This year’s congress (June 16-17) opened with a keynote presentation by Lexi Pappas, who… Read More »