To crack the code of FSHD, patients are absolutely essential
All of the breakthroughs—the discovery of the genetic causes, understanding why some patients vary so greatly in the severity of their symptoms, teasing out the biochemical pathways that could point to future treatments—were made because patients stepped up to the plate.
Too often, we hear patients say they’ll volunteer when there’s a treatment. But we will never get to a treatment unless patients participate in fundamental research now. FSHD is uniquely human, so no laboratory mouse can ever fully model the disease. The genetic “package” that causes FSHD is found only in people. We owe an enormous debt to the patients who give DNA samples. Who submit to long interviews and exhausting physical tests. Allow a surgeon to cut out a small muscle sample. Who fight claustrophobia to lie in the narrow bore of an MRI machine.
Equally important are patients’ family members, both affected and unaffected, who provide the best experimental controls because of their shared genetic and environmental backgrounds. A parent or sibling who has very mild symptoms may hold the key to understanding the factors that protect against the full-blown development of FSHD symptoms in a more severely affected family member.
We are more hopeful today than ever before that a treatment is within sight. We cannot guarantee when that treatment will arrive, but here’s one thing we guarantee: If you volunteer for research, your participation will without question help move us a step closer to that day.
Scientific Overview of FSHD
Read the latest on wikipedia
Glossary of Scientific Terms
FSHD Lab Day was empowering for all
Uniting the FSHD Community in Advancing Research by Nizar Saad, PhD, Columbus, Ohio On July 8th, our lab at Nationwide Children’s Hospital (NCH) in Columbus, Ohio, in collaboration with NCH,… Read More »
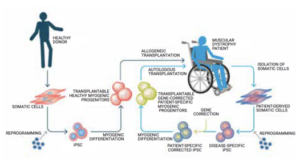
Making strides toward stem cell therapies
University of Minnesota lab reports promising results in mouse FSHD studies by Fred Thys, Weymouth, Massachusetts Work by a team led by Dr. Rita Perlingeiro at the University of Minnesota… Read More »
Arrowhead files for regulatory clearance to start a Phase 1/2 study in FSHD
From Arrowhead Pharmaceuticals PASADENA, Calif.–(BUSINESS WIRE)–Jul. 17, 2023– Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) today announced that it has filed an application for clearance to initiate a Phase 1/2 clinical trial… Read More »
Passion and hope at the IRC
The immeasurable value of face to face meetings by Raj Badiani, FSHD UK I was given the opportunity to attend the FSHD Society’s International Research Congress (IRC) in Milan this… Read More »