FSHD brings with it several important health issues, We’re here to help you learn and understand more. In addition to the information below, follow our blog for regular updates and information on Living with FSHD.
 Anesthetics. By Dr. Halsall and Professor Ellis, Academic Unit of Anesthesia, St. James University Hospital, Leeds, The United Kingdom
Anesthetics. By Dr. Halsall and Professor Ellis, Academic Unit of Anesthesia, St. James University Hospital, Leeds, The United Kingdom
Who should read this?
- All those who have a neuromuscular disorder, even if their symptoms are very mild.
- Everyone who has, or had, a relative with a neuromuscular disorder.
- Professionals involved with the care of people with NMD around operations or treatment under local anesthetics.
Anesthetics People with neuromuscular disorders must take great care if they are to have a local or general anesthetics. Even someone with very mild, or non-existent symptoms, or someone who has a family history of a disorder, needs to let the anesthetist know well in advance so that tests can be carried out and proper care after the operation can be arranged. Many people are afraid of having an anesthetics, mainly through ignorance, but when we look at the rate of complications and even deaths arising from anesthesia we see that it is, in fact, very safe. This safety is the result of a thorough understanding of the patient’s medical condition with a careful assessment before the operation, marked technical improvements in monitoring facilities during the operation, and the provision of good recovery facilities such as High Dependency Units (HDU) and Intensive Care Units (ICU). Patients with neuromuscular disorders (NMDs) deserve special attention when it comes to anesthesia because many of the agents used (gases and chemicals) have effects on both muscle and nervous tissue. The main areas of concern are how the anesthetics agents will affect the muscle and nervous tissue including the heart, which is, itself, a muscle. A skeletal deformity such as scoliosis, or curvature of the spine, can also affect the way the patient responds to anesthesia so it is important to consider that, too.
Anesthetics and the heart. An article printed in the Winter, 1995, No. 20 edition of The Search, showed how people with NMDs could sometimes have associated heart disease. This can occur as a cardiomyopathy, when the heart muscle doesn’t work effectively, or as a defect in the way the electrical activity of the heart is transmitted, a conduction defect. The anesthetics vapors—the smelly agents such as ether and halothane that are inhaled—can reduce the effectiveness of the heart’s muscle contractions and also aggravate any conduction defect. The vapors are all slightly different from each other, some having more effect on the heart than others. So it is important that the anesthetist makes a good assessment of the heart’s condition before the operation which would include the level of physical activity that the patient can manage, and an ECG. Occasionally a more extensive assessment is needed.
Anesthetics and breathing. Doctors need to measure how weak the patient’s muscles are, usually by assessing the amount of physical activity that the patient can perform, and by taking a blood test to measure levels of a muscle enzyme, creatine kinase (CK). Any anesthetics agent that affects the muscles will also affect the muscle we use to breathe. Strong analgesic or sedative agents will affect these muscles indirectly, and muscle relaxants will have a direct effect on them. As breathing (or respiration) may already be difficult for patients with NMDs, these drugs should be used cautiously, and monitoring of breathing after the operation is absolutely essential. As a result, the patient is usually best cared for in a High Dependency Unit or Intensive Care Unit immediately after the operation. The muscles used for swallowing can also be affected which is another reason why good post-operative care is important.
Muscle relaxants. Muscle relaxant drugs should only be used if essential because they tend to have a more profound and prolonged effect in NMD patients compared to other patients. One type of muscle relaxant, called suxamethonium, should usually be avoided. It causes the release of potassium ions (K+) from the muscle tissue into the blood. In normal patients this is usually of little practical significance. In patients with NMD the muscle may normally leak K+ so that a further increase in the levels of K+ in the blood may cause abnormal heart rhythms. A pre-operative blood test to check K+ levels is therefore important.
Local anesthetics. A local anesthetic works by preventing the normal electrical activity in the nerve around which the anesthetics agents are placed. For minor procedures, such as stitches for cuts, they are probably the first choice for patients with NMD because they have few, if any, side effects. However for major local anesthetics techniques, e.g. spinal or epidural, careful assessment of the patient is needed and the type of NMD considered well before the operation.
Changes in body temperature and pre-operative “starvation.” Patients with NMD do not tolerate changes in body temperature or the starvation often associated with anesthesia or surgery as well as normal patients, so steps need to be taken to minimize these problems by keeping the patient warm and well hydrated using drips.
Malignant hyperthermia (MH) and Central Core disease. Malignant hyperthermia (MH) is an inherited disorder, which causes an unexpected, sometimes fatal, reaction in the patient to certain anesthetic drugs. Because some patients with NMD have sometimes experienced similar problems during anesthesia, there have been claims that patients with NMD may also have MH. However, it is generally accepted that the only neuromuscular condition truly related to MH is Central Core Disease (CCD), although this is not always the case. Patients with CCD should be considered potentially susceptible to MH unless proved otherwise by a special type of muscle biopsy, which screens for MH.
To sum up . . .
- Clearly anesthesia for patients with a NMD is not to be undertaken lightly. Such patients should expect the anesthetist to make a careful and thorough assessment of their particular condition and their current state of health.
- They are not suitable to be treated as ‘Day Cases’ because doctors should carry out pre-operative investigations, and enough time and recovery facilities should be available after the operation.
- It is absolutely essential that the person affected by a NMD should inform the anesthetist even if there are only minor symptoms or no symptoms at all. Occasionally a neuromuscular disorder in a person who had no symptoms has come to light only because of an unexpected problem with anesthesia, particularly in young children. The anesthetist should also be warned if there is an inherited NMD in the family.
- If possible, ask for the anesthetist to be forewarned before admission to hospital and consider wearing a Medic Alert bracelet or similar informational device in case of accidents.
- It is always a good idea to make sure hospital staff have copies of “Fact Sheets” about your condition. If you are going to have an anesthetics, you might want to show your anesthetist this article.
* * * * * * * * *
Download this article by a concerned parent: An Overview of Anesthetic Concerns by Raymond Huml, DVM (PDF)
Can Respiratory Insufficiency Occur in FSHD? Yes. Respiratory involvement can be seen. Evaluation of the symptoms and signs of respiratory insufficiency should be sought during routine clinic visits in patients with moderate to severe FSHD. Regular monitoring of respiratory function is suggested, as one might experience insufficiency over a long period of time without presenting signs. Symptomatic respiratory insufficiency can be initially managed with nighttime non-invasive pressure support, for example, a BiPAP machine. In very severe cases, patients may require the use of a ventilator.
In standard practice, trauma (ER, ICU), surgery, and anesthesiology settings, care for FSHD patients with respiratory insufficiency should be taken not to suppress respiratory drive with narcotics unless it is a situation of palliative care. In trauma (ER, ICU), surgery, and anesthesiology settings, it is important to notify the doctors about FSHD and any respiratory problems the patient might have or be at risk for. Download and carry the FSHD Society Medical Alert card.
Oxygen supplementation can be detrimental to FSHD patients with hypercarbic (high CO2) respiratory failure and lead to worsening CO2 levels. Oxygen should generally not be administered unless BiPAP or similar ventilatory support is also being used. Your physician and a pulmonologist can help you periodically monitor CO2 levels when in the office or pulmonary function lab in the hospital.
Pulmonary and Respiratory Health and FSHD. Fatigue is often part of FSHD because the muscles of FSHD patients have to work harder than normal muscles, but fatigue may also have additional causes. Some FSHD patients have been found to have respiratory impairment, including sleep apnea and lower than normal forced vital capacity. The causes have not yet been definitively proven, but many doctors believe that FSHD may be an important factor. If you feel fatigue, discuss it with your doctor. If while sleeping you breathe in a labored manner or momentarily stop breathing, this may indicate sleep apnea or other respiratory impairment; again, discuss it with your doctor. Because some doctors, even experienced neurologists, don’t associate FSHD with respiratory problems, your doctor may be reluctant to order respiratory tests; be persistent if you feel fatigue or sleep problems. The doctor may order breathing tests including forced vital capacity and nocturnal oxymetry tests, and blood tests such as a blood gas test. These are non-invasive tests that generally don’t require a hospital stay. A sleep study is a more complex test that may require an overnight hospital stay. The doctor may prescribe CPAP, BiPAP, or other mechanical ventilation at night to help breathing, increase oxygen, and improve sleep. CPAP, BiPAP, and similar mechanical ventilation devices are small machines that blow air into a patient’s nose, which assists the patient in exhaling. The air is delivered by a hose running from the machine to a mask or similar apparatus around the patient’s nose. The doctor may order the tests to be repeated periodically to ensure that the settings on the machine are appropriate. A respiratory therapist (RT) is a health professional trained in respiratory health and problems. An RT should be part of the team that monitors respiratory health. The doctor and RT may recommend stacked breathing exercises. These exercises involve a facemask attached to a football-shaped plastic bladder. A helper holds the face mask around the mouth of the person doing the exercise. The person inhales rapidly several times in succession without exhaling, while the helper squeezes the bladder to push air into the person’s lungs. This exercises the chest muscles and increases oxygen intake. These exercises don’t require much time, and a family member can help an FSHD patient do them. FSHD patients who can’t walk may be at risk of developing blood clots on long airplane flights. On the advice of their doctors, some people take an anti-blood clotting prescription medication before flights to reduce the risk of clotting. See also the sections on Respiratory Therapists and Pulmonologists.
BreatheNVS is an excellent web resource for management of respiratory issues through noninvasive support. The site is being developed by Dr. John Bach, a professor of Physical Medicine & Rehabilitation, professor of Neurosciences, the director of the New Jersey Medical School Muscular Dystrophy Association Clinic, and medical director of the Center for Ventilator Management Alternatives.
Here is a video of Dr. Bach presenting on noninvasive support for individuals with FSHD.
Links to Recent Articles About Respiratory Problems in FSHD
- 2010 FSH Society International Patient and Researcher Network Meeting, July 30–August 1, 2010. Presentation slides for talk titled: Breathing and Respiratory Health for People with FSHD, by Joshua O. Benditt, MD, Pulmonary and Critical Care Medicine, University of Washington Medical Center, Seattle. Click here to read Abstract.
- Della Marca G, Frusciante R, Vollono C, Dittoni S, Galluzzi G, Buccarella C, Modoni A, Mazza S, Tonali PA, Ricci E. Sleep quality in Facioscapulohumeral muscular dystrophy. J Neurol Sci. 2007 Dec 15;263(1-2):49-53. Epub 2007 Jun 26. Click here to read Abstract.
- Simonds AK. Respiratory complications of the muscular dystrophies. Semin Respir Crit Care Med. 2002 Jun;23(3):231-8. Click here to read Abstract.
- Carter GT, Bird TD. Ventilatory support in facioscapulohumeral muscular dystrophy. Neurology. 2005 Jan 25;64(2):401. Click here to read Abstract.
- Wohlgemuth M, van der Kooi EL, van Kesteren RG, van der Maarel SM, Padberg GW. Ventilatory support in facioscapulohumeral muscular dystrophy. Neurology. 2004 Jul 13;63(1):176-8. Click here to read Abstract.
The information in this section has been reviewed by Joshua Benditt, MD, a board certified physician at the University of Washington Medical Center, where he is medical director of Respiratory Services and a UW professor of Pulmonary, Critical Care and Sleep Medicine.
It is also available as a downloadable PDF.
You have been referred to this page by a person who has a NMD, Facioscapulohumeral muscular dystrophy (FSHD, ICD-10 code G71.02). This individual may have respiratory muscle weakness and may use mechanical/assisted ventilation and other respiratory muscle aids.
- This information is shared in the spirit of positive collaboration and to spread awareness that many treatments that help individuals without NMD may be harmful to individuals with NMD such as FSHD.
- This individual and/or their NMD care team are experienced in NMD respiratory involvement and have learned what does and does not work.
- Check their Medical Alert card for emergency contacts and medical providers.
Emergency Considerations
- Beware of:
- Supplemental oxygen (O2)
- Only administer supplemental O2 for sustained periods with monitoring EtC02 capnograph.
- If given without the individual’s mechanical/assisted ventilation, it can cause:
- Decreased responsiveness;
- Hypercapnia (an increased level carbon dioxide in the blood and lungs);
- Suppressed respiratory drive, which can be life-threatening and cause respiratory arrest.
- General anesthesia medications – e.g. volatile inhalation anesthetics, and depolarizing muscle relaxants.
- Narcotics, sedatives, and opioids can cause - Potentially life-threatening suppression of breathing, especially when mechanical/assisted ventilation is not in use.
- Supplemental oxygen (O2)
General Respiratory Involvement
- Respiratory muscle weakness:
- Affects muscles between ribs, diaphragm, and sometimes bulbar (mouth and throat) muscles.
- Can cause orthopnea (discomfort and difficulty breathing while lying down). A semi-reclined position may be required along with their mechanical ventilation during examination and/or therapy.
- Mechanical/assisted Bi-level ventilation:
- Assists ventilation (movement of air into and out of the lungs);
- Corrects oxygen (O2) and carbon dioxide (CO2) gas exchange abnormalities;
- Can be used continuously via a mask, mouthpiece, or a tracheostomy tube.
- Manual and mechanically assisted cough:
- Assists a weak and/or ineffective cough;
- Clears airway secretions and can prevent infection.
- Narrow, restricted airway can cause:
- Difficult intubation and need for smaller endotracheal tube.
- Reduced lung volume results from:
- Areas of micro-atelectasis (small areas of collapse) in the lungs;
- Skeletal abnormalities such as scoliosis (progressive curvature of the spine).
- Discussion with this individual and his or her NMD care team is encouraged.
- Affects muscles between ribs, diaphragm, and sometimes bulbar (mouth and throat) muscles.
Adapted with permission from Dr. Kofi Boahene’s website. By Kofi Boahene, MD Johns Hopkins Hospital, Baltimore, Maryland Facial expression is an important part of human communication, allowing us to reflect emotions and project nonverbal cues. Small skeletal muscles that mostly originate and connect with the soft tissues of the face are responsible for animating facial expression. The facial muscles also support the facial skin and fat, giving it shape and form. Blinking, whistling, blowing out candles and drinking from a straw are simple day-to-day actions that depend upon the fine action of the facial muscles. These functions are severely affected when the facial muscles become progressively weak as seen in FSH dystrophy. FSHD patients often complain of changes in their speech. They are unable to generate the intraoral pressure to pronounce plosive sounds such as “P” and “B.” As the facial muscles become weaker, mid-facial fat descends, causing the corners of the mouth to turn downwards and giving a falsely sad appearance. In my surgical practice, I have been developing new reconstructive techniques for FSHD patients. Muscle tone and movement are two attributes to consider when exploring treatment options for facial weakness in these patients. Muscles in the body are maintained at a slightly contracted state, which is called the muscle tone. The always activated state of partial contraction keeps the muscles firm, offers some resistance, and maintains form and posture. Facial muscle tone keeps the face from drooping, eyelids from sagging, keeps the lips together, and offers some cheek resistance when preparing to blow a candle. When the facial muscles become dystrophic, they lose tone and become flaccid. Facial tone can be improved through procedures to introduce some degree of form, shape, stiffness, and resistance to the flaccid areas of the weakened face. Collagen matrix grafts strategically placed in the lower eyelid can lift the eyelid vertically up and allow better eyelid closure. Fat grafting to the buccal space over the flaccid buccinator muscle introduces some resistance to the cheek, which helps with plosive sounds. Targeted lip augmentation together with muscle placation techniques can be used to correct lip position, shape, and closure. In the future, regenerative medicine and tissue engineering research may offer new options for reconstituting dystrophic muscle. For facial expression and sphincter function around the eyes and mouth, improving tone is not sufficient. One needs to restore muscle movement. Although the facial nerves are intact, dystrophic facial muscles do not respond well and animate the face in a very limited manner. Replacing a dystrophic muscle is technically feasible using microsurgical techniques. Microsurgery now allows us to borrow a section of muscle from one part of the body and transplant it to another part to generate a desired effect. The main limitation in pursuing muscle replacement surgery as treatment for facial FSHD is the progressive nature of the disease and the number of potential donor muscles that can be affected. In other words, why replace a weak muscle with another muscle that may also become weak? One solution is to determine potential donor muscles that are not commonly affected in FSHD. In the head, the temporalis muscle appears to be spared in FSHD. We have successfully transferred the temporalis muscle with techniques uniquely tailored to FSHD patients. We are investigating other potential donor muscles. The ideal donor muscle should be available for harvest without any significant functional loss and should be spared from progressive dystrophy. Such a muscle can be transferred to the face and re-innervated with the existing facial nerve. The current options for reanimating the face in FSH dystrophy are promising but should be approached carefully under the direction of a multidisciplinary team that specializes in this disease.
 Cardiac complications in facioscapulohumeral muscular dystrophy (FSHD) are rare, and patients in general have normal longevity. This predisposes them to the usual age-related cardiac complications, and management of these problems is the same as in non-dystrophic patients. The presence of cardiac abnormalities in FSHD is debated. While most authors have noted the presence of diverse electrocardiographic abnormalities, some have found no abnormalities on electrocardiography, chest radiography, Holter monitoring, or echocardiography. Nuclear scanning with thallium-201 has demonstrated defects consistent with diffuse fibrosis. Abnormalities in systolic time intervals on echocardiography and elevations in atrial natriuretic peptide are consistent with subclinical cardiomyopathy. Atrial paralysis, an unusual condition in which the atrium is devoid of electrical or mechanical activity, has been reported by several investigators. An in-depth investigation of cases of atrial paralysis found that 33 percent of all cases were associated with Emery-Dreifuss muscular dystrophy (EDMD). The literature states that the finding of atrial paralysis distinguishes EDMD from other similar dystrophies such as FSHD.
Cardiac complications in facioscapulohumeral muscular dystrophy (FSHD) are rare, and patients in general have normal longevity. This predisposes them to the usual age-related cardiac complications, and management of these problems is the same as in non-dystrophic patients. The presence of cardiac abnormalities in FSHD is debated. While most authors have noted the presence of diverse electrocardiographic abnormalities, some have found no abnormalities on electrocardiography, chest radiography, Holter monitoring, or echocardiography. Nuclear scanning with thallium-201 has demonstrated defects consistent with diffuse fibrosis. Abnormalities in systolic time intervals on echocardiography and elevations in atrial natriuretic peptide are consistent with subclinical cardiomyopathy. Atrial paralysis, an unusual condition in which the atrium is devoid of electrical or mechanical activity, has been reported by several investigators. An in-depth investigation of cases of atrial paralysis found that 33 percent of all cases were associated with Emery-Dreifuss muscular dystrophy (EDMD). The literature states that the finding of atrial paralysis distinguishes EDMD from other similar dystrophies such as FSHD.
Links to Recent Articles About Cardiac Involvement in FSHD
- Trevisan CP, Pastorello E, Armani M, Angelini C, Nante G, Tomelleri G, Tonin P, Mongini T, Palmucci L, Galluzzi G, Tupler RG, Barchitta A. Facioscapulohumeral muscular dystrophy and occurrence of heart arrhythmia. Eur Neurol. 2006;56(1):1-5. Epub 2006 Jun 27. Click here to read Abstract.
- Galetta F, Franzoni F, Sposito R, Plantinga Y, Femia FR, Galluzzi F, Rocchi A, Santoro G, Siciliano G. Subclinical cardiac involvement in patients with facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2005 Jun;15(6):403-8. Epub 2005 Apr 21. Click here to read Abstract.
- Emmrich P, Ogunlade V, Gradistanac T, Daneschnejad S, Koch MC, Schober R. Facioscapulohumeral muscle dystrophy and heart disease [Article in German] Z Kardiol. 2005 May;94(5):348-54. Click here to read Abstract.
- Laforêt P, de Toma C, Eymard B, Becane HM, Jeanpierre M, Fardeau M, Duboc D. Cardiac involvement in genetically confirmed facioscapulohumeral muscular dystrophy. Neurology. 1998 Nov;51(5):1454-6. Click here to read Abstract.
 An occupational therapist (OT) is a healthcare professional trained in rehabilitation who helps people learn how to perform the activities of daily life. People whose FSHD has progressed beyond the mild stage can benefit by consulting with an OT about performing daily living activities at home and at work.
An occupational therapist (OT) is a healthcare professional trained in rehabilitation who helps people learn how to perform the activities of daily life. People whose FSHD has progressed beyond the mild stage can benefit by consulting with an OT about performing daily living activities at home and at work.
OTs can help FSHD patients improve function; increase comfort; and reduce stress, fatigue, and risk of injury. Home visits are essential—there is no substitute for having an OT observe the actual conditions of daily living and recommend ways of improving safety, efficiency, comfort, and convenience. Home visits also help caregivers find ways to improve comfort and efficiency and reduce their burden and risk. A joint home visit by an OT and a physical therapist working together can be especially effective.
Some OTs are experts on workplace ergonomics. Many employers are willing to pay for an ergonomics consultation. If an employee has a disability as defined by the Americans With Disabilities Act, the employer may be legally required to do so. Simple and inexpensive modifications in the workplace can often improve the ability of someone with FSHD to perform efficiently, comfortably, and safely.
Using a computer strains and fatigues the hands, wrists, and arms of some people with FSHD. Voice recognition software can reduce strain, conserve energy, and increase efficiency. The leading voice recognition software is Dragon NaturallySpeaking by Nuance.
As people’s FSHD progresses, they may need to use assistive technology and equipment including wheelchairs, scooters, canes, walkers, lifters, reachers, ramps, electric beds, and commode/shower chairs. They may need to modify their homes. OTs are experts in assistive technology and equipment. A good durable medical equipment (DME) dealer will have an OT available to help clients choose the best equipment for their needs. For people who use wheelchairs and scooters, an OT can provide invaluable advice about the most appropriate mobility device and about posture, positioning, and seat cushions.
Attending disability products trade shows (such as the Abilities Expo) is an excellent way to see, try, and compare equipment. In choosing products, technology, and equipment, it’s essential to get advice from sources that are independent from one’s health insurance company.
Some people’s FSHD progresses to the point where they begin to feel uncomfortable or unsafe driving. At this point, it is critical to have their driving evaluated by an adaptive driving expert. Some hospitals have adaptive driving programs that are usually staffed by an OT who is also a certified driving instructor. Evaluations are expensive, and insurance usually doesn’t cover them. But if driving is necessary to maintain employment, the rehabilitation agencies of some states may pay part or all of the cost of an evaluation, adaptive driving equipment, and training. An increasing variety of adaptive driving technology is available, from simple mechanical hand controls to sophisticated electronic controls.
Resources
- For more information about occupational therapy, visit the website of the American Occupational Therapy Association.
- The National Registry of Rehabilitation Technology Suppliers (NRRTS) is a professional organization of wheelchair dealers that provides training and credentialing.
- Many of the most knowledgeable and experienced wheelchair dealers are credentialed by and registered with NRRTS.
- Several organizations sponsor disability products trade shows. The largest is the Abilities Expo, which is held in several cities each year.
- Easter Seals offers useful information about accessibility, equipment, and home modifications.
- To learn more about Dragon NaturallySpeaking voice recognition software, visit Nuance Communications.
A majority of FSHD patients report pain, which often can be chronic. Pain may be related, at least in part, to overuse of muscles and fatigue, and is a leading reason patients seek physical therapy. Not many studies have been done about pain in FSHD.
Whether and how to treat pain are decisions for each patient in consultation with his or her doctor. For a discussion about treating pain, download our Physical therapy and exercise brochure, written by Katie Eichinger, PhD, and Shree Pandya, PT, MS, and published by the FSH Society. Our FSHD University webinar on pain describes the multidisciplinary theory of pain and pain management.
The following is an abstract of the study by R. Ted Abresch of the University of California, Davis, on FSHD and pain as presented at the FSH Society Facioscapulohumeral Muscular Dystrophy International Research Consortium 2007.
* * * * * * * * *
Chronic Pain in Persons With Facioscapulohumeral Dystrophy and Other Neuromuscular Disorders, by R. Ted Abresch, Department of Physical Medicine and Rehabilitation, UC Davis Davis California 95616 USA
Introduction Recent preliminary research suggests that pain may be a significant problem for many persons with FSHD. For example, Bushby et al. recently reported on four individuals with FSHD who identified pain as their most disabling symptom and complained of between three to seven separate pain complaints. In addition, our group found that 83 percent of a sample of 811 individuals with various neuromuscular diseases (NMDs), including 64 persons with FSHD, reported at least some ongoing pain problems. Moreover, the frequency and severity of pain in their combined sample of patients with FSHD, MMD, and a sample of patients with limb-girdle syndrome was significantly greater than levels of pain reported by the general US population.
In a more recent study, our group surveyed 193 individuals with a variety of NMDs, including 18 patients with FSHD and 26 patients with MMD, and found that 73 percent of the sample as a whole (89 percent of patients with FSHD and 69 percent of those with MMD) reported pain problems, with 27 percent of the overall sample reporting severe pain (19 percent of patients with FSHD and 50 percent of patients with MMD).
We found that pain was reported to interfere moderately with a number of activities of daily living across all of the NMD diagnostic groups (range of interference ratings, 2.6 to 4.63 on 0-10 interference ratings scales) and to occur all over the body (least common, abdomen/pelvis at 16 percent; most common, back at 49 percent). However, we were unable to examine pain interference, pain sites, and pain treatments as a function of the diagnostic group due to the low sample sizes of the individual NMD diagnostic groups in our previous study.
Although the preliminary findings from our group and others indicate that chronic pain can be a serious problem for many persons with FSHD, much remains unknown about the nature and scope of pain in these patient populations. Importantly, most of the research on pain that has been performed with patients with FSHD has reported findings from a mixed population of patients with limited sample sizes for particular diagnoses. This limits both the reliability and generalizability of the available findings.
Descriptive analyses regarding pain with larger samples of patients with specific diagnoses would provide for greater reliability of the findings and would allow us to confirm (or question) previously published data concerning pain in patients with these conditions. Moreover, because FSHD is a progressive disease, it is possible that the onset of pain, and the severity of pain once it develops, is related to a patient’s age. This study sought to address the need for more information about the nature and scope of pain in persons with FSHD and myotonic muscular dystrophy.
Methods Retrospective, cross-sectional study performed using a community-based survey. Participants were recruited from the NIH-funded National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy Patients and Family Members (n = 296); the University of Washington NMD Clinic list (n = 87); the Quality of Life Pediatric Survey Study (n = 8); and four participants who independently contacted study personnel. A total of 296 potential subjects with MMD or FSHD contacted us. Of these, 235 (93 percent) completed and returned a mail survey questionnaire on the nature and scope of their pain. The survey included questions asking about demographic information, NMD-related information, pain intensity, pain interference, pain location, and pain treatments. All participants provided basic demographic information about their gender, age, race/ethnicity, educational level, marital and employment status. They also provided information about their NMD diagnosis, including approximate date of diagnosis, type of physician who made the diagnosis, whether or not they had received a DNA confirmation of diagnosis, and their use of assistive devices for ambulation. Average pain intensity over the past week was assessed using an 11-point numerical rating scale (0 = “no pain” to 10 = “pain as bad as could be”) taken from the Grading of Chronic Pain scale (GCP). Pain interference with daily activities was assessed using a 12-item interference scale adapted from the Brief Pain Inventory Pain Interference scale (BPI). Participants were asked to indicate whether or not they experience bothersome pain in one or more of 17 specific body sites (head, neck, shoulders, upper back, lower back, arms, elbows, wrists, hands, buttocks, hips, chest, abdomen/pelvis, legs, knees, ankles, and feet). Participants were asked to indicate if they were currently using or had ever used any of 25 specific pain treatments (physical therapy, nerve blocks, biofeedback/relaxation training, acupuncture, magnets, massage, hypnosis, counseling/psychotherapy, mexiletine, Neurontin®, tricyclic antidepressants, narcotics/opioids, acetaminophen, aspirin/ibuprofen, valium, Tegretol®, baclofen, TENS units, anticonvulsants, chiropractic adjustments, heat, ice, marijuana, strengthening exercises or range of motion exercises).
Results/Discussion More individuals with FSHD (82 percent) than with MMD (60 percent) reported pain. The most frequency of pain sites for both diagnostic groups were lower back (66 percent MMD, 74 percent FSHD) and legs (60 percent MMD, 72 percent FSHD). Moreover, the average pain severity reported in patients with FSHD in our sample (4.40 out of 10 in the current sample) and percent of patients with FSHD who report severe pain (23 percent in the current sample) also replicate previous findings. These pain problems are chronic with a mean duration of pain being 11-13 years in our samples.
This finding, when considered in light of both the high frequency of pain in general and the existence of subgroups of patients (about 25 percent in both samples) who report severe pain, underscores the need to identify and provide effective pain treatments for patients with these neuromuscular diseases.
Both FSHD and MMD patients endorsed generally similar levels of interference of pain with functioning, although there was a slight trend for patients with MMD (range of interference ratings, 2.14 to 4.17/10) to report higher levels of interference with some activities than patients with FSHD (range, 1.14 to 3.65/10). Pain was reported to have a moderate degree (3.73 and 3.53/10) of interference with enjoyment of life. Moreover, the strength of the associations found between pain severity and interference with the life activities tended to be strong (correlation coefficients greater than .50 for six of the 12 activities; the correlation coefficient was never less than .30).
Modern biopsychosocial pain rehabilitation treatments focus not only on the pain itself, but also on the extent to which pain interferes with function. The significant pain interference reported by the patients in this study, when considered in light of the multi-domain focus of contemporary pain treatments, raises the possibility that patients with neuromuscular disease and chronic pain might benefit from pain rehabilitation approaches.
Overall, the sites of pain reported by these patients reflect the body areas that are commonly affected by these NMDs (e.g., low back and legs as most common; chest, buttocks, and head as relatively less common). The most frequent pain site for both diagnostic groups was the low back. This reflects the fact that low back pain is a common site of pain in the able-bodied adult population.
In both FSHD and MMD the degree of back pain may be exacerbated by the fact that the trunk and neck flexors are among the weakest muscle groups in both of these disorders. Moreover, in both diseases there is a significant imbalance between the extensors and flexors of the neck and the trunk. As the individuals become weaker, the biomechanical stresses are increased and pain may become more pronounced. This is supported by the fact that subjects with FSHD reported a significantly older age at which pain began in their hands and ankles compared to the subjects with MMD.
No single treatment for pain has been shown to be widely effective for subjects with FSHD and MMD. No treatment was currently used by more than 46 percent of all of the patients reporting pain, or by more than 42 percent of the patients reporting severe pain. The most common treatments were ibuprofen or aspirin (used by 46 percent of patients with pain), acetaminophen (used by 34 percent), and strengthening exercises (used by 29 percent). Of those treatments that had been tried, the most effective (rated as providing at least 5/10 relief) were ibuprofen/aspirin, opioids, massage, chiropractic manipulation, nerve blocks, heat, and marijuana.
However, it should also be noted that many of these treatments also have significant drawbacks. For example, opioids, which were rated as the most effective (6.49/10) in this sample, had been tried by 25 percent of the sample, but were only currently being used by 8 percent of the sample. These data suggest that the pain relief gained from the opioids did not outweigh their side effects (grogginess and constipation) when taken at the doses required to provide substantial relief. Similarly, marijuana, although reported to be highly effective (6.00/10), was still used by only a little over half of the patients who had tried it (4 percent of the sample using, 7 percent had tried). The significant side effects (such as decreased motivation) and significant problems with access may decrease the desirability of this treatment.
The other treatments that were rated as being relatively highly effective tend be short lasting. This may explain the fact that many of the patients who had ever tried massage, chiropractic manipulation, and nerve blocks no longer receive these treatments. The only treatment that was relatively highly effective and was still being used by a substantial number of patients (26 percent) was heat. Perhaps this is because heat is an extremely accessible treatment (most people own a hot water bottle or heating pad) that has few, if any, negative side effects.
Overall, the findings suggest that there remain too few options for pain relief for patients with MMD and FSHD and chronic pain. There is a substantial need for the development of effective and long-lasting pain treatments for persons with MMD and FSHD that can be made easily available and that have few negative side effects.
Pain is likely related, at least in part, to fatigue. Our results are consistent with a recent study of NMD patients that included 139 subjects with FSHD and 322 subjects with MMD. Severe fatigue was reported by 61-74 percent of these patients and the severity of the fatigue was correlated with an increase in the number of problems with physical functioning, mental health, and bodily pain. Although the causal relationship is not clear, it is likely that physical disability leads to both pain and fatigue conjointly, but chronic pain would certainly worsen fatigue symptoms.
Conclusion The findings from this study indicate that pain is a common problem in both FSHD and MMD, with the majority of adults with these conditions reporting pain. The most frequent pain sites for both diagnostic groups were lower back and legs. Significant differences between diagnostic groups in frequency of pain at specific sites were found in shoulders, hips, and feet, with participants with FSHD reporting pain more often in their shoulders and hips, and participants with MMD reporting pain more often in their feet and hands. These findings highlight the need to identify and provide effective pain treatments for patients with FSHD and MMD.
Future work needs to address chronic pain in a variety of other neuromuscular diseases.
 A physical therapist (PT) who is experienced with FSHD patients can be invaluable, but they are few and far between. You may need to train your own PT. Find a physical therapist who is willing to learn about FSHD, who will take the time to understand the unique ways in which your body is affected and can work with you to develop exercises that will keep unaffected muscles health and strong. Give the PT a copy of our Physical Therapy brochure.
A physical therapist (PT) who is experienced with FSHD patients can be invaluable, but they are few and far between. You may need to train your own PT. Find a physical therapist who is willing to learn about FSHD, who will take the time to understand the unique ways in which your body is affected and can work with you to develop exercises that will keep unaffected muscles health and strong. Give the PT a copy of our Physical Therapy brochure.
Also visit our FSHD University page about Exercise and Fitness for videos and blog posts.
Physical therapists administer some exercises directly (e.g., stretching) and teach patients how to perform other exercises; for the latter, the goal for patients is to learn the exercises so they can eventually do them on their own or with the help of family members or friends.
FSHD patients ask their doctors to prescribe physical therapy when they feel their FSHD is progressing more rapidly than usual; periodically to monitor how they perform their exercises; and to rehabilitate specific conditions or injuries such as strained or pulled muscles or injuries from falls. Many find it useful to go to a PT for a “tune-up” from time to time.
Many people with FSHD enjoy swimming and find it beneficial. Swimming has a low impact on the joints and bones and a low risk of injury. The buoyancy of the water provides resistance, which affords the opportunity for gentle exercise and also enables some people to do things they are unable to do on land—for example, someone who can’t stand or walk on land may be able to stand and walk in water. It can also be beneficial to do stretching exercises in the water.
Watsu®, a form of massage done in the water, combines the benefits of ordinary massage and being in the water. Both massage and Watsu should be done only by certified professionals. For FSHD patients with reduced mobility, being in the water can provide freedom of movement and a welcome feeling of exhilaration, energy, and relaxation. In recent years there have been an increased number of therapeutic swimming pools with access and special programs for disabled people; some of these pools have extremely warm water, which can be soothing for people who can’t move quickly or who are in pain.
For some people with FSHD who can no longer walk, standing with the aid of a standing frame can be beneficial. A standing frame is a piece of equipment that can straighten and elevate people into a standing position and support them in that position. For some people, standing in a standing frame can help maintain range of motion and reduce the possibility of contractures, relieve pressure on the posterior, increase circulation, reduce stress, realign the internal organs, and provide weight bearing to preserve bones.
As with all exercise, it’s important for FSHD patients to consult with their doctors and physical therapists before beginning a swimming program and before using a standing frame. The risk of overuse is ever present, so it’s essential to monitor closely how you feel. When in doubt about an exercise, seek professional help. When in pain, stop doing it.
Download our Physical Therapy for FSHD brochure, written by Shree Pandya, PT, DPT, MS, and Kate Eichinger, PhD, PT, DPT, NCS, and published by the FSHD Society.
Links to Recent Publications on FSHD and Physical Therapy and Exercise
- 2010 FSH Society International Patient and Researcher Network Meeting, July 30—August 1, 2010. Presentation slides for talk titled: Physical Activity and Exercise: A Physician’s and a Patient’s Perspectives, by Craig M. McDonald, MD, PhD, RRTC/NMD Director Rehabilitation Research and Training Center in Neuromuscular Diseases, University of California, Davis, and Nils Hakansson, Patient. Click here to read Abstract
- Pandya S, King WM, Tawil R. Facioscapulohumeral dystrophy. Phys Ther. 2008 Jan;88(1):105-13. Epub 2007 Nov 6. Click here to read Abstract.
- van der Kooi EL, Kalkman JS, Lindeman E, Hendriks JC, van Engelen BG, Bleijenberg G, Padberg GW. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol. 2007 Jul;254(7):931-40. Epub 2007 Mar 14. Click here to read Abstract.
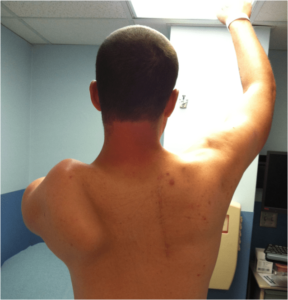 One of the most common and problematic symptoms of FSHD is scapular “winging,” which is caused by weakness in the scapular stabilizer muscles that hold the scapula (shoulder blade) in place. Some patients significantly lose their ability to raise the arm above the shoulder and, consequently, major functional impairment in critical activities such as eating, drinking, lifting, and reaching. Scapular fixation is a surgical procedure that stabilizes the scapula by attaching it to the rib cage to prevent it from “winging.” This procedure comes in more than one variety. One method is the scapular fusion, in which a piece of bone is taken from the patient, typically from the pelvic bone, and used to fuse the shoulder blade to the rib cage. Another method involves using screws or wires, rather than a bone graft, to hold the scapula in place. Scapular fixation typically involves general anesthesia and requires the patient to be in a cast after the surgery. The universe of potential candidates for scapular fixation is small, including some people with FSHD and a limited number of those with other conditions. The procedure is extremely specialized. Few are performed each year compared to most orthopedic procedures, and there are very few orthopedic surgeons with significant experience. The decision to consider this surgery is very complex and highly individualized. Some FSHD patients who have had it have experienced significantly improved shoulder function. As with all elective surgeries, however, trade-offs are involved. Things to consider include the risks typically associated with surgery and anesthesia in general, the possible reduction of patients’ ability to move their arms behind their back, the effect of reduced mobility and prolonged inactivity during the recuperation period, and the demands of rehabilitation both while the cast is on and after it has been removed. Patients who have had this procedure recommend only considering a surgeon with significant experience in performing the procedure; discussing in great depth with the surgeon one’s lifestyle, work and family demands, and physical abilities and limitations; coordinating closely with one’s neurologist; and speaking with FSHD patients who have had the procedure. If the decision is made to have the procedure, the rehabilitation program requires careful planning with the surgeon and with physical and occupational therapists with relevant experience, and the patient must be rigorous and diligent in doing the rehabilitation.
One of the most common and problematic symptoms of FSHD is scapular “winging,” which is caused by weakness in the scapular stabilizer muscles that hold the scapula (shoulder blade) in place. Some patients significantly lose their ability to raise the arm above the shoulder and, consequently, major functional impairment in critical activities such as eating, drinking, lifting, and reaching. Scapular fixation is a surgical procedure that stabilizes the scapula by attaching it to the rib cage to prevent it from “winging.” This procedure comes in more than one variety. One method is the scapular fusion, in which a piece of bone is taken from the patient, typically from the pelvic bone, and used to fuse the shoulder blade to the rib cage. Another method involves using screws or wires, rather than a bone graft, to hold the scapula in place. Scapular fixation typically involves general anesthesia and requires the patient to be in a cast after the surgery. The universe of potential candidates for scapular fixation is small, including some people with FSHD and a limited number of those with other conditions. The procedure is extremely specialized. Few are performed each year compared to most orthopedic procedures, and there are very few orthopedic surgeons with significant experience. The decision to consider this surgery is very complex and highly individualized. Some FSHD patients who have had it have experienced significantly improved shoulder function. As with all elective surgeries, however, trade-offs are involved. Things to consider include the risks typically associated with surgery and anesthesia in general, the possible reduction of patients’ ability to move their arms behind their back, the effect of reduced mobility and prolonged inactivity during the recuperation period, and the demands of rehabilitation both while the cast is on and after it has been removed. Patients who have had this procedure recommend only considering a surgeon with significant experience in performing the procedure; discussing in great depth with the surgeon one’s lifestyle, work and family demands, and physical abilities and limitations; coordinating closely with one’s neurologist; and speaking with FSHD patients who have had the procedure. If the decision is made to have the procedure, the rehabilitation program requires careful planning with the surgeon and with physical and occupational therapists with relevant experience, and the patient must be rigorous and diligent in doing the rehabilitation.
For more information about scapular surgery and related considerations, watch this recent conversation Young Adults: Jan. 15, 2024 Special Round Table on Scapular Fixation Surgery>>
For more information about scapular fixation, download our Physical therapy and exercise brochure, written by Katy Eichinger, PhD, and Shree Pandya, PT, MS, and published by the FSH Society. See also the section on Orthopedic Surgeons.
For general information about orthopedic surgery, see the following websites:
- American Academy of Orthopaedic Surgeons
- American Shoulder and Elbow Surgeons
- The Pediatric Orthopaedic Society of North America
Links to Recent Articles About Scapulothoracic Fusion in FSHD
- 2010 FSH Society International Patient and Researcher Network Meeting, July 30–August 1, 2010. Presentation slides for talk titled: Scapular Fixation Surgery for People with FSHD, by Leigh Ann Curl, MD, Orthopedic Surgery, Sports Medicine, Knee and Shoulder, Harbor Hospital, Baltimore, Maryland. Click here to read Abstract.
- Diab M, Darras BT, Shapiro F. Scapulothoracic fusion for facioscapulohumeral muscular dystrophy. J Bone Joint Surg Am. 2005 Oct;87(10):2267-75. Click here to read Abstract.
- Mummery CJ, Copeland SA, Rose MR. Scapular fixation in muscular dystrophy. Cochrane Database Syst Rev. 2003;(3):CD003278. Click here to read Abstract.

